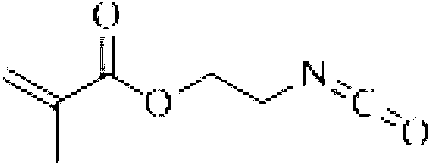Method for modifying acrylic ester by using organic silicon
An acrylate and organosilicon technology, applied in the field of ultraviolet light curing, can solve the problems of increasing difficulty in product separation and purification, dark metal catalyst color, inconvenient experimental operation, etc., to achieve good surface and mechanical properties, improved surface gloss, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 35.86g of 3-aminopropyltrimethoxysilane into a 250ml three-neck round bottom flask, stir electrically, and take an oil bath at 30°C, and take another 56.46g of isocyanatoethyl acrylate, add it slowly, and then heat up to 80°C for reaction 12h.
Embodiment 2
[0018] Add 44.28g of 3-aminopropyltriethoxysilane to a 250ml three-neck round bottom flask, stir electrically, and take an oil bath at 50°C, and take another 62.06g of isocyanatoethyl methacrylate, drop it slowly, and continue the reaction for 24 hours after the drop is completed .
Embodiment 3
[0020] Add 102.78g of bis(-γ-trimethoxysilylpropyl)amine to a 250ml three-necked round-bottomed flask, stir electrically, and take an oil bath at 70°C, and take another 42.35g of isocyanatoethyl acrylate, slowly add it dropwise, and drop it completely Afterwards, the temperature was raised to 90°C for 8 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com