Use of two (quinazolin-4-yl) diselenide compounds in the preparation of anticancer drugs
A kind of technology of bisquinazoline and compound, applied in the field of anticancer biological activity of antitumor drug diselenide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, the synthesis of two (quinazolin-4-yl) diselenides.
[0021] In a three-necked flask equipped with a reflux condenser and a thermometer, add 0.51g (2.5mmol) Na 2 Se 2 Add 0.83g (5.0mmol) 4-chloroquinazoline in batches in the ethanol solution of the above, add in about 1 hour, continue reflux reaction 17 hours, cooling, adjust system pH value with glacial acetic acid to be 5, reaction solution precipitation, use DMF -H 2 O(V DMF :V H2O =2.5:1) Recrystallized to obtain 0.46 g of orange-red solid, m.p.: 260-262°C, yield 44.2%. IR (KBr) v: 3140.8, 3055.6 (v Ar-H ), 1617.2-1466.4 (quinazoline skeleton vibration), 838.2 (δ Ar-H ) cm -1 . 1 H NMR (DMSO- d 6 , 600 MHz) δ: 8.61 (t, J =7.8Hz, 2H, quinazoline H-7), 8.21 (t, J =7.8Hz, 2H, quinazoline H-6), 7.99 (d, J =8.4Hz, 2H, quinazoline H-5), 7.76 (d, J =8.4Hz, 2H, quinazoline H-8), 7.66 (s, 2H, quinazoline H-2). 13 C NMR (DMSO- d 6 , 150 MHz) δ: 127.6 (quinazoline C-5), 129.5 (quinazoline C-6), 1...
Embodiment 2
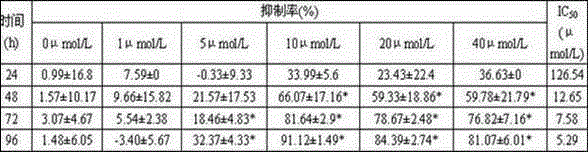
[0022] Example 2. Determination of inhibition of bis(quinazolin-4-yl)diselenide on the proliferation of human breast cancer cell line MDA-MB-435.
[0023] Test method: the drug was dissolved in DMSO to prepare various concentrations, and each concentration was repeated three times; MDA-MB-435 cells were digested to make a suspension 4×10 4 cells / mL, take 10 mL and add it to a large petri dish, and after 24 hours adhere to the wall, add the drug treatment; after 24 hours, randomly take 2 dishes to take pictures and record the state of the cells; suck out the original medium and replace it with the drug-containing medium (10 %FBS 1640) for 72 hours; add 1.5 mL of trypsin, digest for 4 minutes, add the original drug-containing medium to stop digestion, mix well, count the number of cells, take the average value, and calculate the inhibition rate.
[0024] Test results: After testing, when the concentration of bis(quinazolin-4-yl)diselenide was 10 μmol / L, the inhibition rate of MD...
Embodiment 3
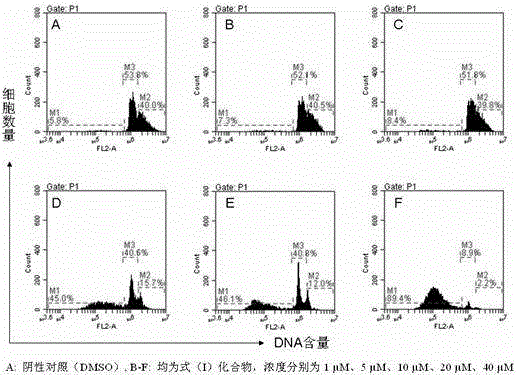
[0025] Example 3. The morphological effects of bis(quinazolin-4-yl)diselenide on human breast cancer cell MDA-MB-435 for 12, 36 and 72 hours.
[0026] Test method: After the test cells were treated with DMSO (negative control), oxaliplatin (positive control), and the compound of formula (I), they were placed in a 96-well plate. Under L, the drug acts for 12 hours, 36 hours, and 72 hours, and the changes in cell morphology are observed with an inverted microscope at 100 times magnification.
[0027] Test results: It can be seen from the morphological observation that when the concentration of bis(quinazolin-4-yl) diselenide is 10 μmol / L, the effect on MDA-MB-435 cells after 12, 36, and 72 hours, The cell morphology changed significantly, which was more significant than that of the positive control drug oxaliplatin, showing extremely high anticancer activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com