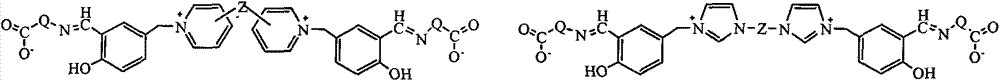Functional ligand and preparation method thereof
A technology of functions and ligands, which is applied in the field of preparation of organometallic coordination polymers, organic ligands, and functional ligands supported by gemini quaternary ammonium salts. It can solve the problems affecting the slow process of organic ligands and metal ion self-assembly, Difficult to cultivate coordination polymers, poor water solubility of aromatic acid ligands, etc., to achieve the effect of abundant raw materials, novel structures, and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
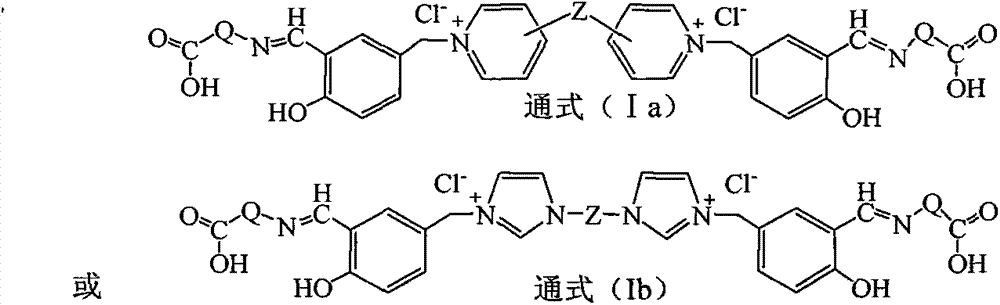
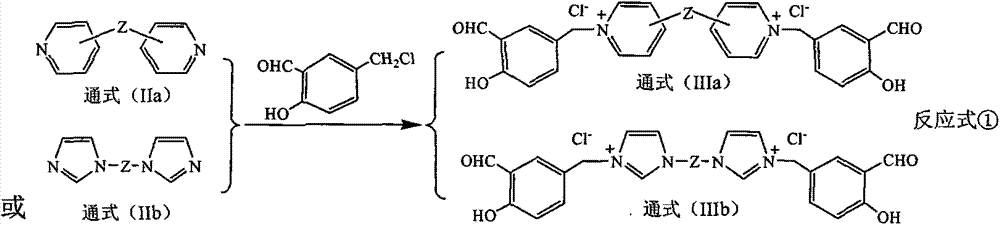
[0032] Example 1 Gemini quaternary ammonium salt supported bis (salicylaldehyde amino acid) functional ligand (Ia-1) Preparation Step 1 Preparation of double salicylaldehyde modified gemini quaternary ammonium salt IIIa-1
[0033]
[0034]In a four-neck flask equipped with a stirrer, weigh 32 grams of 4,4'-bipyridine and dissolve it in 80 ml of acetonitrile to obtain an acetonitrile solution of 4,4'-bipyridine, and then weigh 5-chloro 70 grams of methyl salicylaldehyde were dissolved in 100 milliliters of acetonitrile to prepare an acetonitrile solution of 5-chloromethyl salicylaldehyde. Control the temperature of the acetonitrile solution of 5-chloromethyl salicylaldehyde between 8 and 15°C, slowly add the acetonitrile solution of 4,4'-bipyridine into the 5-chloromethyl salicylaldehyde under stirring In the acetonitrile solution, after reacting for 4 hours, raise the temperature of the reaction material to 80-85°C, then stir the reaction for 10 hours, lower the temperature...
Embodiment 2
[0039] Example 2 Preparation of Gemini quaternary ammonium salt supported bis(salicylaldehyde amino acid) functional ligand (Ia-2)
[0040]
[0041] According to the method and operation steps of Example 1, the anthranilic acid in Step 3 of Example 1 was replaced with glycine to obtain light yellow Ia-2.
[0042] Dissolve the light yellow Ia-2 in deionized water, adjust the pH to 8.5 with sodium carbonate aqueous solution, slowly add copper chloride aqueous solution dropwise, the color of the solution gradually turns dark blue, and blue crystals precipitate out after a few days.
Embodiment 3
[0043] Example 3 Preparation of Gemini quaternary ammonium salt supported bis(salicylaldehyde amino acid) functional ligand (IIa-1)
[0044]
[0045] According to the method and operation steps of Example 1, N, N, N', N'-tetramethylethylenediamine in Step 3 of Example 1 was replaced with 1,4-diimidazolidine to obtain orange-red IIa -1.
[0046] Dissolve orange-red IIa-1 in deionized water, adjust the pH to 8.5 with sodium carbonate aqueous solution, slowly add zinc chloride aqueous solution dropwise, the color of the solution gradually deepens, and red crystals precipitate out after a few days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com