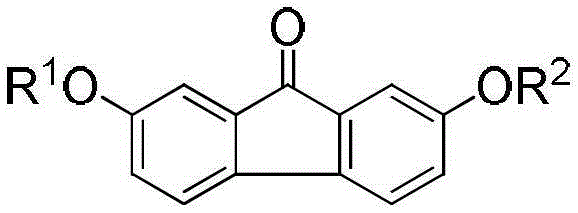
2, 7-bi-substituted fluorenone derivative and preparation method and application thereof
A derivative, two-substituted technology, applied in the field of medicinal chemistry, can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
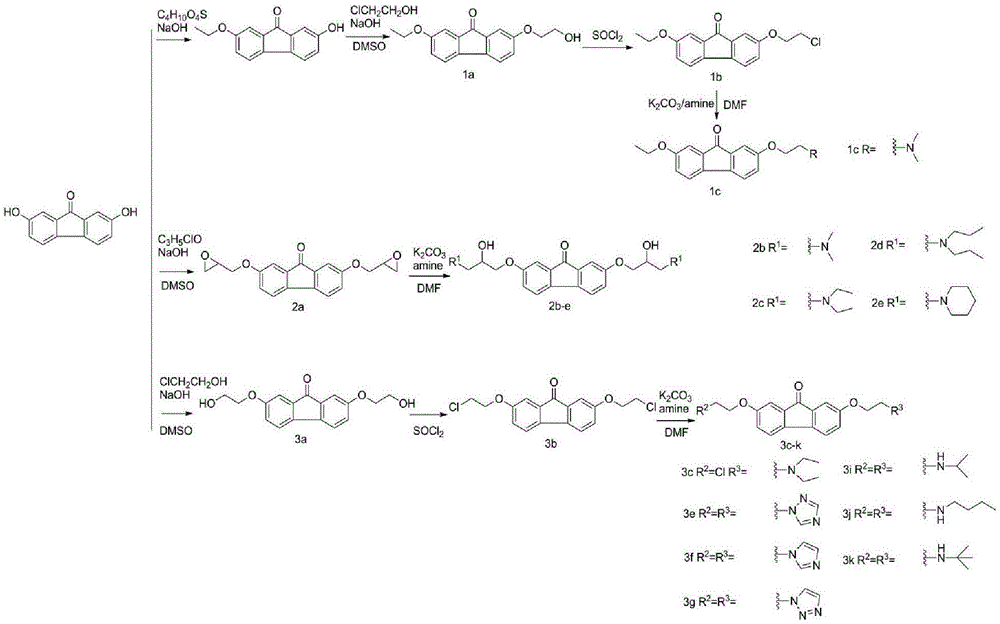
Examples
Embodiment 1
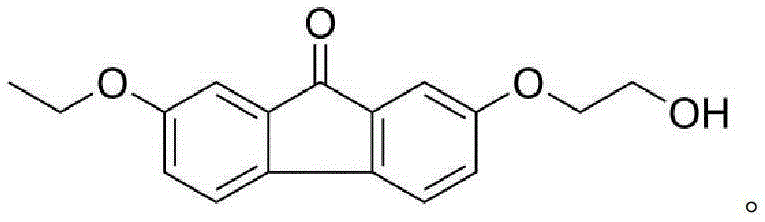
[0029] Example 1: Synthesis of 2-ethoxy-7-(2-hydroxyethoxy)-fluorenone (1a)
[0030] Add 2,7-dihydroxyfluorenone (10g, 0.047mol) into an aqueous solution of 200ml NaOH (4g, 0.1mol), stir until dissolved, add diethyl sulfate (9.2ml, 0.07mol) dropwise to the system, and react 5 After hours the precipitate was collected by filtration. The precipitate was dissolved in 80ml of DMSO, NaOH (2g, 0.05mol) and chloroethanol (4.1ml, 0.06mol) were added, and reacted overnight at 70°C. Pour the reaction system into 1M NaOH aqueous solution, collect the precipitate and wash and dry it, then dissolve the precipitate in 50ml CH 2 Cl 2 , the insoluble matter was filtered off and recrystallized in isopropanol to obtain 2-ethoxy-7-(2-hydroxyethoxy)-fluorenone. Orange solid, 30% yield. m.p.158-160°C.1H NMR (400MHz, CDCl3) δ: 7.27(s,1H),7.25(s,1H),7.13(dd,J=4.4,2.4Hz,2H),6.93(ddd,J= 10.4,8.2,2.4Hz,2H),4.12–4.08(m,2H),4.05(q,J=7.0Hz,2H),3.98(s,2H),1.42(t,J=7.0Hz,3H). 13C NMR(101MHz,CDCl3)δ:19...
Embodiment 2
[0031] Example 2: Synthesis of 2-ethoxy-7-(2-chloroethoxy)-fluorenone (1b)
[0032] 2-Ethoxy-7-(2-hydroxyethoxy)-fluorenone (2g, 0.007mol) was dissolved in 40ml of thionyl chloride and reacted at 60°C, monitored by TLC until the reaction was completed. The reaction system was poured into 1M NaOH aqueous solution, the precipitate was collected, washed and dried to obtain a crude product. Column chromatography gave 2-ethoxy-7-(2-chloroethoxy)-fluorenone. Orange solid, 90% yield. m.p.146.6-147.4°C.1H NMR(400MHz,CDCl3)δ:7.23(d,J=2.3Hz,1H),7.21(d,J=2.3Hz,1H),7.08(d,J=1.9Hz,2H ),6.88(ddd,J=14.3,8.1,2.5Hz,2H),4.19(t,J=5.8Hz,2H),3.99(q,J=7.0Hz,2H),3.75(t,J=5.8Hz ,2H),1.35(t,J=7.0Hz,3H).13C NMR(101MHz,CDCl3)δ:196.44,159.50,158.50,138.30,137.14,136.03,135.95,121.19,120.97,120.70,120.62,110.319,1 ,68.48,64.02,41.76,14.76.Anal.Calcd.for C17H15ClO3:C67.44,H4.99;Found C67.41,H5.01.MS(ESI):m / z[M-Cl]+calcd267.4 , found 267.3.
Embodiment 3
[0033] Example 3: Synthesis of 2-ethoxy-7-(2-dimethylaminoethoxy)-fluorenone (1c)
[0034] Dissolve 2-ethoxy-7-(2-chloroethoxy)-fluorenone (0.4g, 0.0013mol) in 40mlDMF, add potassium carbonate (0.5g, 0.0036mol) and aqueous dimethylamine (33% , 1.5ml, 0.01mol), 70 ° C for 24 hours, with saturated saline and CH 2 Cl 2Extract and separate the crude product by column chromatography to obtain 2-ethoxy-7-(2-dimethylaminoethoxy)-fluorenone. Orange solid, 85% yield. m.p.80-81.6°C.1H NMR (400MHz, CDCl3) δ: 7.19(d, J=8.1Hz, 2H), 7.06(d, J=7.0Hz, 2H), 6.89(d, J=8.0Hz, 1H ),6.84(d,J=8.0Hz,1H),4.03(t,J=5.4Hz,2H),3.98(dd,J=13.8,6.9Hz,2H),2.70(t,J=5.3Hz,2H ),2.30(s,6H),1.34(t,J=6.9Hz,3H).13C NMR(101MHz,CDCl3)δ:193.77,159.35,159.08,137.72,137.31,135.95,135.93,120.99,120.85,120.56, 120.49,110.19,110.14,66.31,63.98,58.11,45.77,14.76.Anal.Calcd.for C19H21NO3:C73.29,H6.80N4.50;Found C73.24,H6.82,N4.51.MS(ESI) :m / z[M+H]+calcd312.2,found312.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com