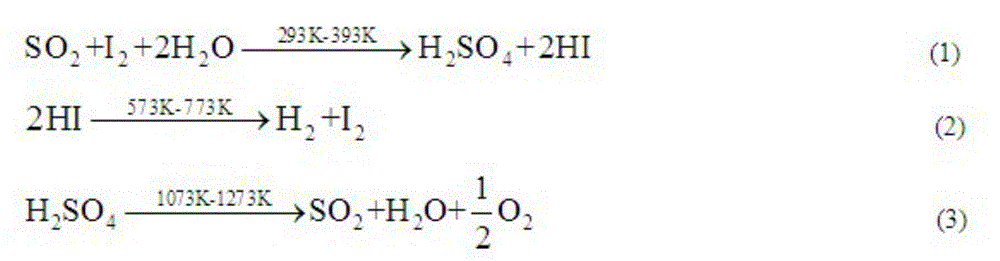Method for promoting bunsen reaction in thermochemical sulfur-iodide cycle hydrogen production
A thermochemical and reaction technology, applied in the direction of hydrogen production, etc., can solve the problems of low HI concentration in HIx phase and slow reaction kinetic rate, and achieve the effect of improving kinetic rate and omitting the HI concentration step.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
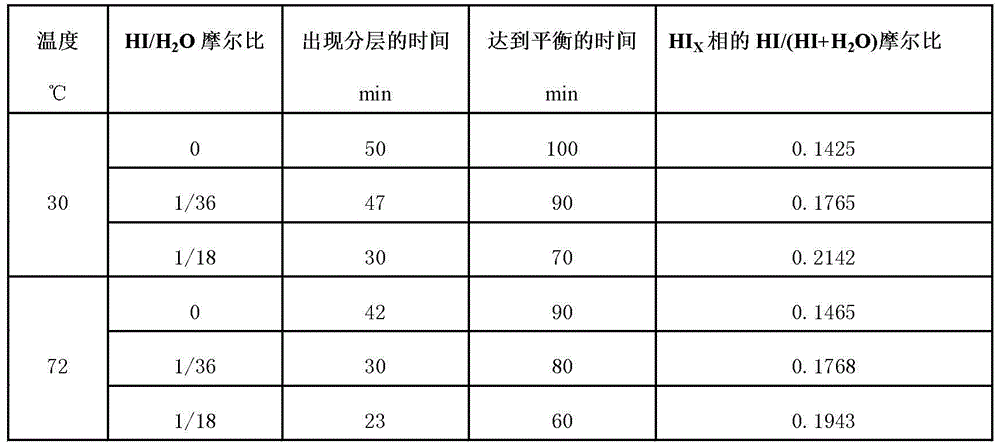
[0022] 0.78mol I 2 and 4.2mol H 2 O was added to the reactor, and the reaction solution was heated to a set temperature of 30°C while stirring the reaction solution at a constant speed; SO 2 Pass the above reaction solution at a flow rate of 60NmL / min, at this time, the Bunsen reaction will proceed spontaneously to generate H 2 SO 4 and HI; in the presence of excess iodine, the resulting mixed solution undergoes liquid-liquid phase separation at a certain moment, forming a layered H 2 SO 4 phase (upper layer) and HIx phase (lower layer), the reaction reaches a liquid-liquid equilibrium state after a period of time.
specific Embodiment 2
[0023] 0.78mol I 2 , 0.117mol HI and 4.2mol H 2 O is added to the reactor, the reaction solution is heated to a set temperature of 30°C, and the reaction solution is stirred at a constant speed to ensure that I 2 Fully dissolved; SO 2 Pass the above reaction solution at a flow rate of 60NmL / min, at this time, the Bunsen reaction will proceed spontaneously to generate H 2 SO 4 and HI; in the presence of excess iodine, the resulting mixed solution undergoes liquid-liquid phase separation at a certain moment, forming a layered H 2 SO 4 phase (upper layer) and HIx phase (lower layer), the reaction reaches a liquid-liquid equilibrium state after a period of time.
specific Embodiment 3
[0024] 0.78mol I 2 , 0.233mol HI and 4.2mol H 2 O is added to the reactor, the reaction solution is heated to a set temperature of 30°C, and the reaction solution is stirred at a constant speed to ensure that I 2 Fully dissolved; SO 2 Pass the above reaction solution at a flow rate of 60NmL / min, at this time, the Bunsen reaction will proceed spontaneously to generate H 2 SO 4 and HI; in the presence of excess iodine, the resulting mixed solution undergoes liquid-liquid phase separation at a certain moment, forming a layered H 2 SO 4 phase (upper layer) and HIx phase (lower layer), the reaction reaches a liquid-liquid equilibrium state after a period of time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com