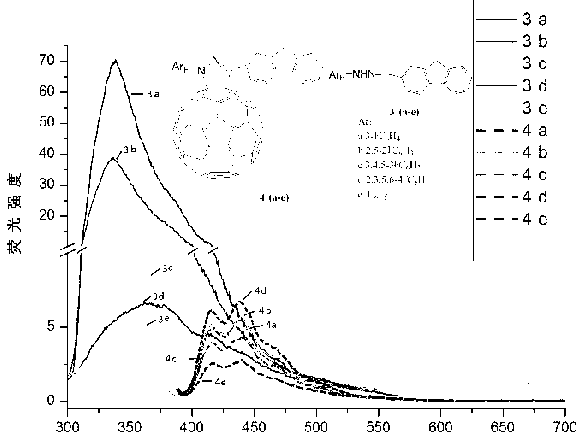
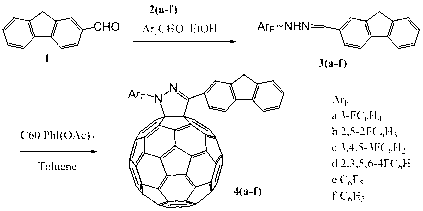
1-(N-fluorinated phenyl)-3-fluorenyl pyrazoline fullerene C60 and preparation method thereof
A technology of fluorenylpyrazoline fullerene and fluorine-containing phenyl, which is applied in the field of 1--3-fluorenylpyrazoline fullerene C60 and its preparation, and can solve the problems affecting the fluorescence effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Add 0.2 g of 2-fluorenaldehyde and 0.11 g of phenylhydrazine to a round bottom flask, use ethanol as the solvent, react at 70°C for half an hour, cool, filter and take the filter cake, and recrystallize to obtain 1-(N-phenyl) -3-Fluorenylhydrazone. Add 1-(N-phenyl)-3-fluorenylhydrazone 0.03g, C 60 0.036 g and 0.032 g of iodobenzene acetate were reacted at 40° C. for 5 hours with toluene as a solvent, and spin-dried to obtain a crude product. The crude product is separated by column chromatography with toluene / carbon disulfide as the eluent to obtain the target product 1-(N-phenyl)-3-fluorenylpyrazoline fullerene C 60 . Brown solid, yield: 33(70)%.
[0019] IR (KBr, cm-1): v 3442, 2920, 2851, 1592, 1490, 1027, 577, 525.
[0020] 1 H NMR (500 MHz, CS 2 – CDCl 3 ): δ 8.43 (s, 1H), 8.30 (d, J = 8.0 Hz, 1H), 7.94 (d, J = 7.5 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.77 (d, J = 7.5 Hz, 1H), 7.54 (d, J = 7.5 Hz, 1H), 7.45 (t, J = 8.0 Hz, 1H), 7.37 (t, J = 7.5 Hz,...
Embodiment 2
[0022] Example 2: Add 0.2 g of 2-fluorenaldehyde and 0.13 g of 3-fluorophenylhydrazine into a round-bottomed flask, use ethanol as the solvent, react at 70°C for half an hour, cool, filter to take the filter cake, and recrystallize to obtain 1'-(N -3-fluorophenyl)-3'-fluorenylhydrazone. Add 1'-(N-3-fluorophenyl)-3'-fluorenyl hydrazone 0.032g, C 60 0.036 g and 0.032 g of iodobenzene acetate were reacted at 40° C. for 5 hours with toluene as a solvent, and spin-dried to obtain a crude product. The crude product was separated by column chromatography using toluene / carbon disulfide as the eluent to obtain the target product 1'-(N-3-fluorophenyl)-3'-fluorenylpyrazoline fullerene C 60 . Brown solid, yield: 34(72)%.
[0023] IR (KBr, cm-1): v 3453, 2921, 2851, 1605, 1578, 1047, 573, 525.
[0024] 1 H NMR (500 MHz, CS 2 – CDCl 3 ): δ 8.41 (s, 1H), 8.28 (d, J = 7.5 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.78 (d, J = 7.5 Hz, 1H), 7.70 (td, J = 2.0 Hz, J = 1...
Embodiment 3
[0027] Example 3: Add 0.2 g of 2-fluorenaldehyde and 0.15 g of 2,5-difluorophenylhydrazine into a round-bottomed flask, use ethanol as the solvent, react at 70°C for half an hour, cool, filter the filter cake, and recrystallize to obtain 1' -(N-2,5-Difluorophenyl)-3'-fluorenylhydrazone. Add 1'-(N-2,5-difluorophenyl)-3'-fluorenylhydrazone 0.034g, C 60 0.036 g and 0.032 g of iodobenzene acetate were reacted at 40° C. for 5 hours with toluene as a solvent, and spin-dried to obtain a crude product. The crude product was separated by column chromatography using toluene / carbon disulfide as the eluent to obtain the target product 1'-(N-2,5-difluorophenyl)-3'-fluorenylpyrazolinefullerene C 60 . Brown solid, yield: 33(70)%.
[0028] IR (KBr, cm-1): v 3443, 2920, 2851, 1727, 1615, 1500, 1048, 574, 525.
[0029] 1 H NMR (500 MHz, CS 2 – CDCl 3 ): δ 8.36 (s, 1H), 8.24 (d, J = 8.0 Hz, 1H), 7.82 (d, J = 8.0 Hz, 1H), 7.74 (d, J = 7.5 Hz, 1H), 7.56-7.53 ( m, 1H),7.51 (d, J = 7.0 Hz, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com