Anti-liver cancer whole cell vaccine modified by hbx and its preparation method and application
A whole cell vaccine, liver cancer cell technology, applied in the field of anti liver cancer whole cell vaccine, can solve the problems of weak immunogenicity and difficulty in causing immune response, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of the anti-hepatoma whole cell vaccine of embodiment 1 HBx gene modification
[0051] Hepa1-6 (mouse hepatoma cells, purchased from ATCC) were inoculated in a culture dish with a density of 70-80% and in the logarithmic growth phase, using a non-replicating recombinant adenovirus vector carrying the HBx gene (structure and construction process) Refer to reference 1 and reference 2 for the construction process of AdHBx, the nucleotide sequence of its expression cassette is shown in Seq ID NO.3) to infect cells with a multiplicity of infection (MOI) of 20, discard the virus after 2 hours of virus infection, Change to fresh DMEM medium containing 10% fetal bovine serum and continue culturing for 24 hours, digest with 0.25% trypsin, collect cells by centrifugation, wash 3 times in serum-free DMEM medium, resuspend the cell pellet with an appropriate amount of cell culture medium, adjust the cell density into 1×10 7 individual / mL. The treated cells were irradi...
Embodiment 2
[0052] Example 2 In vivo anti-tumor experiment of the HBx gene-modified anti-hepatoma whole cell vaccine of the present invention
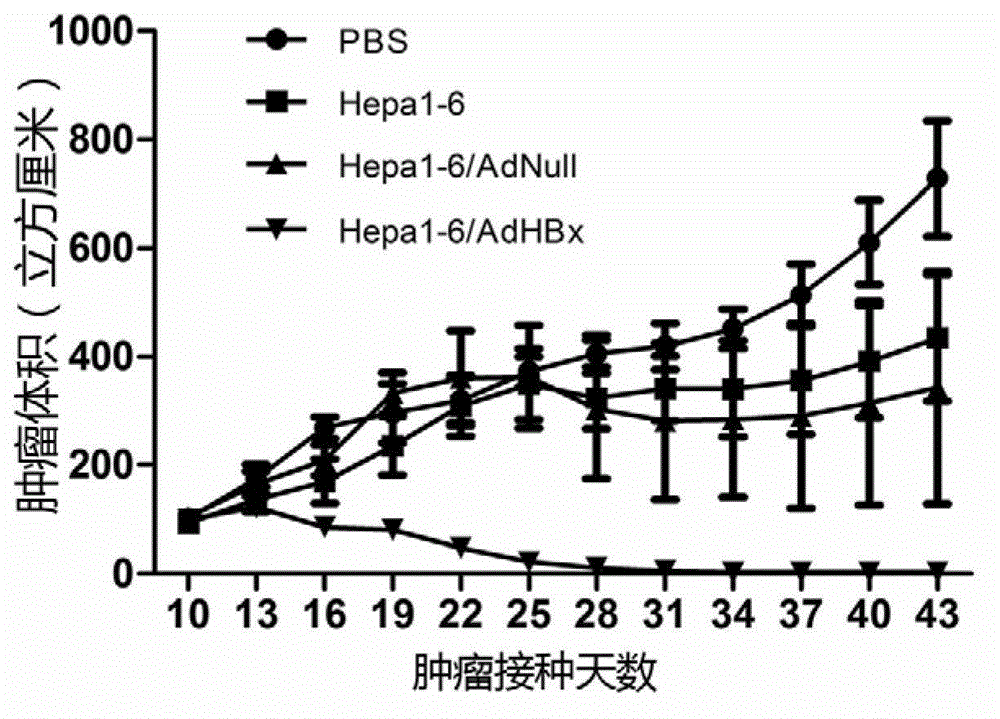
[0053] Will 2.5×10 6 Hepa1-6 / HBx mouse liver cancer cells were inoculated subcutaneously on the right flank of 6-8 week-old C57 mice (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.). When the tumor diameter reached 3 mm, the mice were randomly Divide into 4 groups, 6 mice in each group, perform 1×10 on the left side of the back of the mice 6 Cells / vaccine injection (recorded as day 0), boosted once on day 14 and day 21 respectively. After immunization, the long diameter (a) and wide diameter (b) of the tumor were measured every two days with a vernier caliper, with (a×b 2 )×0.52 to calculate the tumor volume and draw the tumor growth curve. see results figure 1 .
[0054] figure 1 Show: There are significant differences in the tumor volumes of mice in each group ( figure 1 , P3 , the tumors of the remaining fi...
Embodiment 3
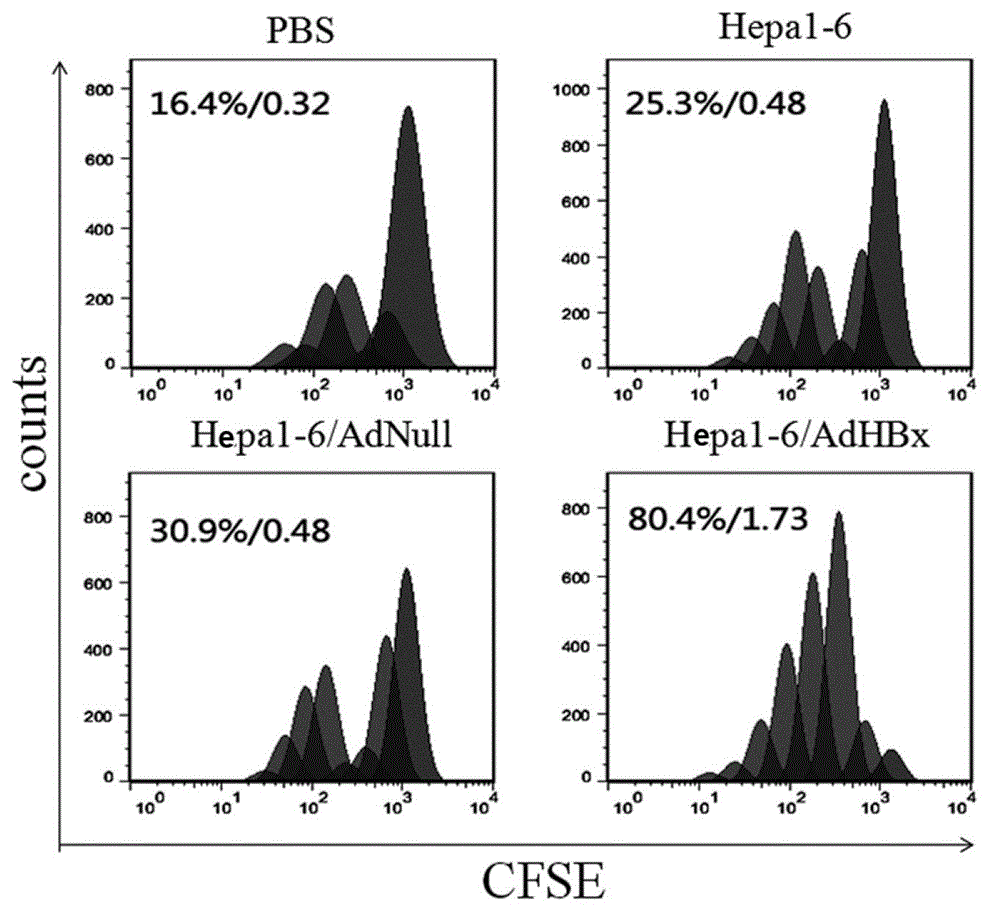
[0055] Example 3 The anti-tumor mechanism of the anti-hepatoma whole cell vaccine with HBx gene modification of the present invention
[0056] 1. T lymphocyte typing
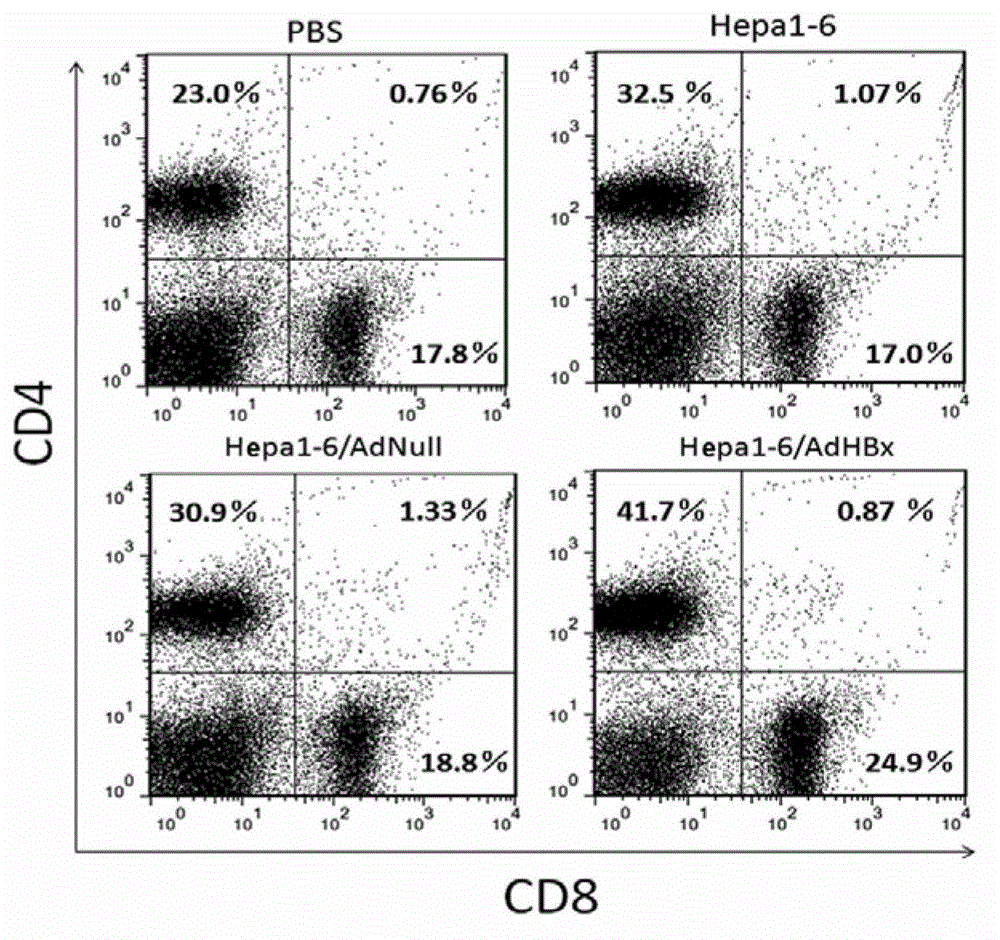
[0057] One week after the last booster immunization in Example 2, the mice were sacrificed by cervical dislocation, the spleen was aseptically removed, ground and filtered on a 200-mesh metal sieve, the suspension was collected, lysed with erythrocyte lysate, and washed three times with PBS. Take 10 for each sample 5 Add fluorescent dye-labeled anti-CD4 (purchased from Biolegend, H129.19) and anti-CD8 antibody (purchased from Biolegend, 53-6.7) to each cell, label at 4°C for 30 min, wash three times with PBS, and use a flow cytometer (purchased From BD Biosciences, FACSVerse). see results figure 2 .
[0058] figure 2 In the middle, lymphocytes were isolated after three times of immunotherapy, and CD4 and CD8 were labeled with fluorescent dyes, and flow cytometric detection was performed. The results show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com