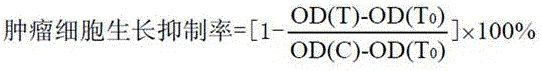Camptothecin derivatives and their antitumor applications
A kind of derivative, the technology of camptothecin, which is applied to the new camptothecin derivative and its anti-tumor application field, can solve the problems such as toxic reaction and decreased drug activity, and achieve the effects of low cost, easy availability of raw materials and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1. Preparation of side chain 5,6-dihydronorcantharidin monomethyl ester 2
[0031] 1) Preparation of 5,6-didehydronorcantharidin 1:
[0032] Take out a certain amount of maleic anhydride from the reagent bottle, place it in a dry grinding body and grind it finely, then weigh 12.021g of the finely ground maleic anhydride with an electronic balance, put it in a dry three-necked flask, and plug it Stopper, add diethyl ether and stir, when the volume of diethyl ether is 90 mL, the maleic anhydride is completely dissolved. After the maleic anhydride was completely dissolved, 13 mL of furan was slowly added with a dropping funnel for 13 minutes (minutes, also abbreviated as min). The temperature was controlled to start the reaction at 38°C. After reacting for 1 hour (hour, also abbreviated as h), white solids appeared in the solution, and the longer the time, the more white solids there were. After reacting for 24 hours, suction filtration was performed to obta...
Embodiment 2
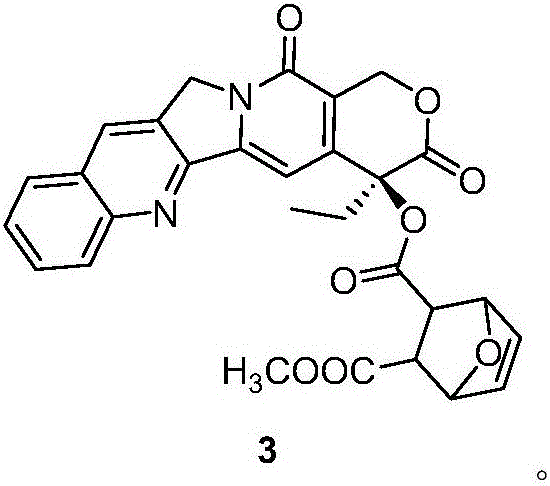
[0036] Embodiment 2. Preparation of camptothecin derivative 3
[0037] .
[0038]1.2 g (3.48 mmol) camptothecin was dissolved in 20 ml chloroform, and 5,6-dihydronorcantharidin monomethyl ester 2 (1.38 g, 7 mmol), 1-(3-dimethylaminopropyl base)-3-ethylcarbodiimide hydrochloride (abbreviated as EDCI; 3.2 g, 16.8 mmol) and 4-dimethylaminopyridine (abbreviated as DMAP; 240 mg, 2.44 mmol). React at room temperature for 10 hours. Add 30 ml of chloroform, extract and wash with water, saturated sodium carbonate solution, and saturated saline, respectively, and dry the organic layer with anhydrous magnesium sulfate. The solvent is spin-dried and separated by column chromatography to obtain a yellow solid (1.62 g, 89%). 1 HNMR (CDCl 3 ) : δ 8.40(s, 1H), 8.20(d, 1H), 7.94(d, 1H), 7.83(t, 1H), 7.67(t, 1H), 7.19(s, 1H), 6.95(q, 2H ),5.72(d, 1H), 5.42(d, 1H), 5.29(s, 2H), 3.83(s, 3H), 2.95(s, 2H), 2.88(s, 2H),2.17-2.38(m, 2H), 1.01 (t, 3H). 13 CNMR (CDCl 3 ) :δ 167.13, 164.98, 1...
Embodiment 3
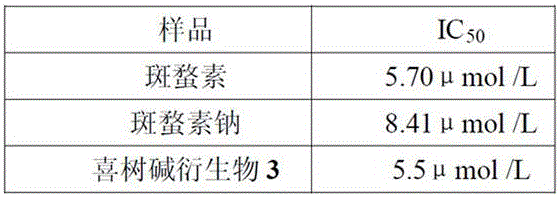
[0039] Embodiment 3, activity test of novel camptothecin derivative
[0040] Using sulforhodamine (sulforhodamine B, SRB) staining.
[0041] When inoculating cells, each cell was inoculated in two 96-well plates in parallel, one was the control plate (T0) and the other was the experimental plate. After culturing in a CO2 incubator for 20 h, the control plate (T0) was taken out, fixed with 50% trichloroacetic acid (TCA), and tested. The test compound was added to the test plate (final concentrations were 5, 2.5, 1.25, 0.625, 0.313 μg·mL-1), and a negative control group (C), an experimental group (T), and a solvent control group were set up. Set 5 duplicate wells in each group, take out the culture plate after continuing to culture for 48 h, fix with pre-cooled 50% TCA (final concentration: 10%), place in 4 °C refrigerator for 1 h, rinse with deionized water, and dry naturally. Stain with 100 μL of 0.4% SRB, rinse with 0.1% acetic acid after 10 min, dry in the air, and final...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com