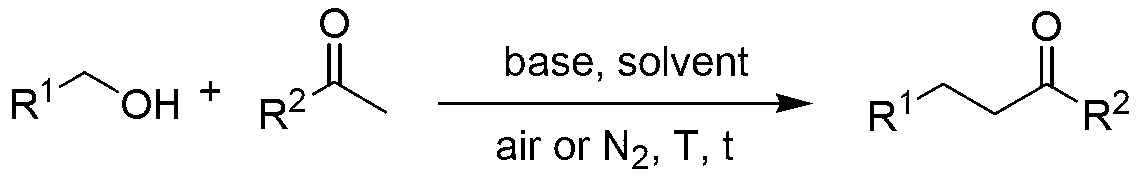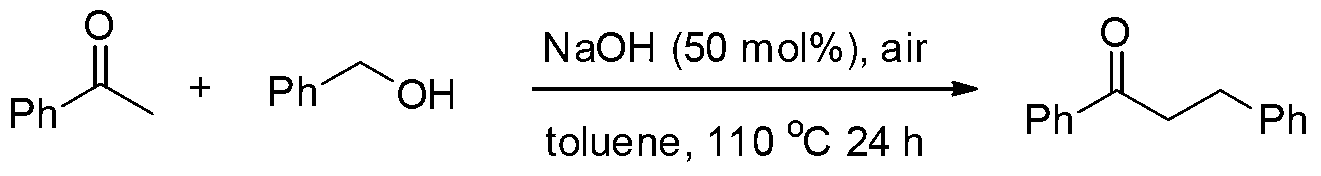Green synthesis method for substituted ketone
A green synthesis and ketone-replacing technology, which is applied in chemical instruments and methods, carbon-based compound preparation, organic compound preparation, etc., can solve problems such as difficult to achieve large-scale application, limited product application, high catalyst price, etc., and achieve good promotion and application potential, ease of operation, and high product recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Preparation of 1,3-diphenyl-1-propanone from acetophenone and benzyl alcohol
[0020]
[0021] Add acetophenone (2mmol), benzyl alcohol (2.4mmol, 1.2equiv.), NaOH (50mol%) in turn into a 100mL reaction tube, and then add 2mL toluene as a solvent, and heat it to 110°C under the air for 24h. 1 HNMR records that the reaction conversion rate is 56%. The product was separated and purified by column chromatography, and the separation yield was 50%. 1 HNMR (500MHz, CDCl 3 ):δ7.94-7.92(m,2H),7.53-7.50(m,1H),7.43-7.40(m,2H),7.29-7.23(m,4H),7.20-7.17(m,1H),3.26 (t,J=7.5Hz,2H),3.05(t,J=7.5Hz,2H). 13 CNMR (125.4MHz, CDCl 3 ): δ199.1, 141.2, 136.7, 132.9, 128.5, 128.4, 128.3, 127.9, 126.0, 40.3, 30.0.
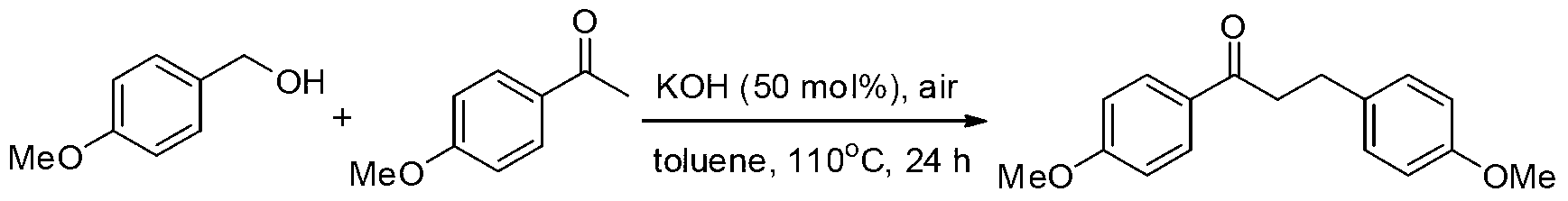
example 2
[0023] Preparation of 1,3-bis(4-methoxyphenyl)-1-propanone from 4-methoxyacetophenone and 4-methoxybenzyl alcohol
[0024]
[0025] Add 4-methoxyacetophenone (2mmol), 4-methoxybenzyl alcohol (2.4mmol, 1.2equiv.), KOH (50mol%) in turn into a 100mL reaction tube, then add 2mL toluene as a solvent, and seal it under air Heating to 110°C for 24h, the reaction conversion rate was 95% as measured by GC-MS. The product was separated and purified by column chromatography, and the separation yield was 75%. 1 HNMR (300MHz, CDCl 3 ):δ7.94(d,J=8.7Hz,2H),7.18(d,J=8.4Hz,2H),6.92(d,J=8.7Hz,2H),6.85(d,J=8.4Hz,2H ),3.85(s,3H),3.78(s,3H),3.22(t,J=7.5Hz,2H),3.00(t,J=7.5Hz,2H). 13 CNMR (125.4MHz, CDCl 3 ):δ197.9,163.3,157.8,133.3,130.2,129.8,129.2,113.8,113.6,55.3,55.1,40.2,29.3.MS(EI):m / z(%)77(14),92(7),107 (7), 108(7), 121(49), 134(12), 135(100), 136(9), 270(29).
example 3
[0027] Preparation of 3-(4-methoxyphenyl)-1-phenyl-1-propanone from acetophenone and 4-methoxybenzyl alcohol
[0028]
[0029] Add acetophenone (2mmol), 4-methoxybenzyl alcohol (2.4mmol, 1.2equiv.), KOH (50mol%) in turn into a 100mL reaction tube, then add 2mL toluene as a solvent, seal and heat to 110°C under air to react After 24 hours, the conversion rate of the reaction was 98% as measured by GC-MS. The product was separated and purified by column chromatography, and the separation yield was 68%. 1 HNMR (300MHz, CDCl 3 ):δ7.97(d,J=7.5Hz,2H),7.57(t,J=7.2Hz,1H),7.46(t,J=7.5Hz,2H),7.19(d,J=8.7Hz,2H ),6.86(d,J=8.7Hz,2H),3.80(s,3H),3.28(t,J=7.5Hz,2H),3.03(t,J=7.5Hz,2H). 13 CNMR (125.4MHz, CDCl 3 ): δ199.4, 158.0, 136.9, 133.3, 133.0, 129.3, 128.6, 128.0, 113.9, 55.3, 40.7, 29.3. MS (EI): m / z (%) 77 (32), 91 (10), 105 (39 ),108(17),121(100),122(10),135(13),240(37).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com