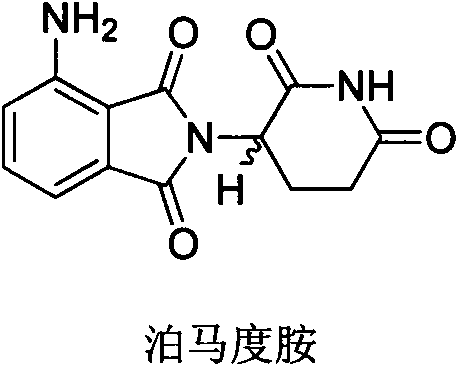
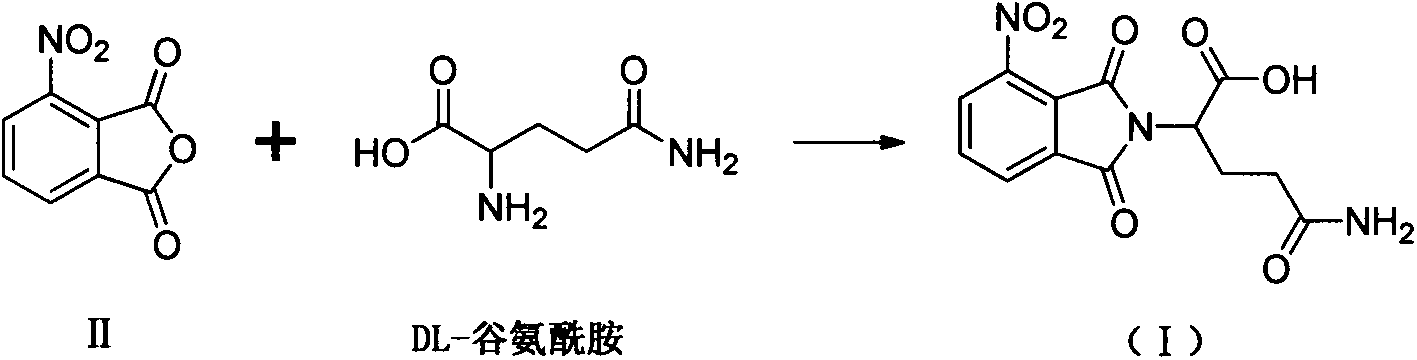
Method for preparing pomalidomide key intermediate
A technology of pomalidomide and intermediates, applied in the field of preparation of N--DL-glutamine, which can solve the problem of difficult price of DL-glutamine tert-butyl ester, low reaction yield and low total yield etc. to achieve good yield, simple reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1N-(3-nitrophthaloyl)-DL-glutamic acid (IV)
[0041] Add 3-nitrophthalic anhydride (II) (10.05g, 0.052mol), DL-glutamic acid (III) (9.26g, 0.063mol) and dry DMF100mL to the reaction flask, react at 86°C for 6h, TLC Monitor the reaction for completion. The reaction solution was cooled to room temperature and concentrated under reduced pressure to obtain an oil. Add 80 mL of water to the oily substance, and a large amount of solid precipitates out under stirring. After filtering, the filter cake is washed with water several times, and dried to obtain a crude product. Add 100 mL of ethyl acetate to the crude product, stir for 6 h, filter, wash the filter cake with ethyl acetate, and dry to obtain 13.56 g of light yellow solid (IV), mp 195-197°C, yield: 81%.
[0042] 1 H-NMR (DMSO-d 6 )δ: 8.33 (d, 1H), 8.22 (d, 1H), 8.10 (t, 1H), 4.86-4.82 (dd, 1H), 2.38-2.21 (m, 4H).
[0043] ESI-MS (m / z): 321 [M-H] -
Embodiment 2
[0044] The preparation of embodiment 2N-(3-nitrophthaloyl)-DL-glutamic anhydride (V)
[0045] Add N-(3-nitrophthaloyl)-DL-glutamic acid (IV) (12.14g, 0.031mol) and 60mL acetic anhydride to the reaction flask, heat to reflux temperature, react for 5.5h, and monitor by TLC The reaction is complete. The reaction solution was cooled to room temperature, a large amount of solids precipitated, filtered, the filter cake was washed with ether, and dried to obtain 10.53 g of off-white solid (V), mp 235-237°C, yield: 92%.
[0046] 1 H-NMR (DMSO-d 6 )δ: 8.37(d, 1H), 8.26(d, 1H), 8.13(t, 1H), 5.54-5.48(dd, 1H), 3.18-2.94(m, 2H), 2.65-2.50(m, 1H) , 2.22-2.10 (m, 1H).
Embodiment 3
[0047] The preparation of embodiment 3N-(3-nitrophthaloyl)-DL-glutamine (I)
[0048] Add N-(3-nitrophthaloyl)-DL-glutamic anhydride (V) (9.87g, 0.032mol), ammonium acetate (4.63g, 0.06mol) and 75mL of glacial acetic acid to the reaction flask, heat up To 55°C, the reaction was stirred for 6h, and the reaction was completed as monitored by TLC. The reaction solution was cooled to room temperature and concentrated under reduced pressure to obtain an oil. Add 60mL of water to the oil, stir for 6h, a large amount of solid precipitates, filter and dry to obtain the crude product. Add 80 mL of ethyl acetate to the crude product, stir for 8 hours, filter, wash the filter cake with ethyl acetate, and dry to obtain 8.76 g of off-white solid (I), mp 194-196°C, yield: 84%.
[0049] 1 H-NMR (DMSO-d 6 )δ: 13.24 (brs, 1H), 8.34 (d, 1H), 8.22 (d, 1H), 8.11 (t, 1H), 7.15 (s, 1H), 6.68 (s, 1H), 4.80-4.77 (dd , 1H), 2.36-2.13 (m, 4H).
[0050] ESI-MS(m / z): 322[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com