Homogeneous catalytic preparation method of gamma-valerolactone
A homogeneous catalysis and valerolactone technology, which is applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions of γ-valerolactone, difficulty in large-scale production, and large amount of catalyst, so that the amount of catalyst can be recycled and the reaction conditions Mild, low-catalyst effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
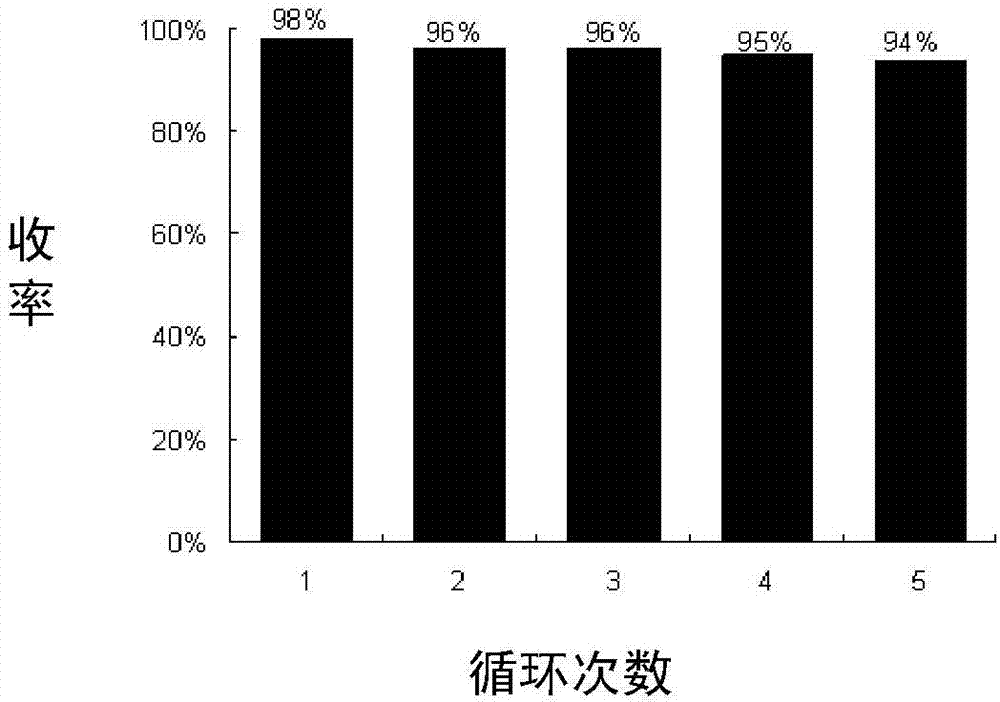
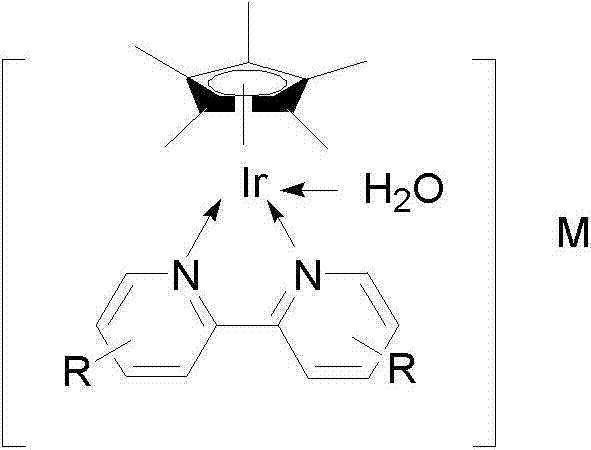
Image
Examples
Embodiment 1-8
[0045] Add 4mL of pre-prepared levulinic acid aqueous solution (1.25mmol / mL) and 1mL of the above-mentioned catalyst 1-8 aqueous solution (500μmol / L) into the reaction kettle respectively, and replace the inside of the reaction kettle with hydrogen gas for 3 times and reduce the hydrogen pressure Adjusted to 1MPa, stirred and reacted at 120°C for 4h. After the reaction was completed, the reaction system was cooled to room temperature, and then the reaction solution was sampled and diluted with methanol, and the yield of the product γ-valerolactone was detected by GC. The catalyst used in the reaction and its molar amount relative to the raw material levulinic acid, the type and amount of the hydrogen source raw material, and the yield results of the reaction are shown in Examples 1-8 in Table 1.
[0046] Finally, the prepared product can be easily obtained, for example, by extractive isolation using ethyl acetate.
Embodiment 9
[0048] Add 4mL of pre-prepared levulinic acid aqueous solution (1.25mmol / mL) and 1mL of the above-prepared catalyst 6 aqueous solution (50μmol / L) into the reactor, and replace the inside of the reactor with hydrogen for 3 times, then adjust the hydrogen pressure to 1MPa , The reaction was stirred at 120°C for 36h. After the reaction was completed, the reaction system was cooled to room temperature, and then the reaction solution was sampled and diluted with methanol, and the yield of the product γ-valerolactone was detected by GC. The catalyst used in the reaction and its molar amount relative to the raw material levulinic acid, the type and amount of the hydrogen source raw material, and the reaction yield results are shown in Example 9 in Table 1.
[0049] Finally, the prepared product can be easily obtained, for example, by extractive isolation using toluene.
Embodiment 10-13
[0051] Add 4 mL of pre-prepared levulinic acid aqueous solution (1.25 mmol / mL), 1 mL of the above-prepared catalyst 6 aqueous solution (500 μmol / L) and 7.5 mmol of formic acid into the reaction kettle, and stir the reaction at 120 ° C for 4 h (wherein Example The reaction time in 13 is 6h). After the reaction was completed, the system was cooled to room temperature, the reaction solution was sampled and diluted with methanol, and the yield of the product γ-valerolactone was detected by GC. For the catalyst used in the reaction and its molar amount relative to the raw material levulinic acid, the type and amount of the hydrogen source raw material, and the reaction yield results, refer to Examples 10-13 in Table 1.
[0052] Finally, the prepared product can be easily obtained, for example, by extractive isolation using benzene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com