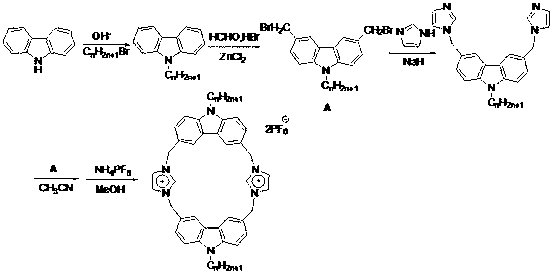
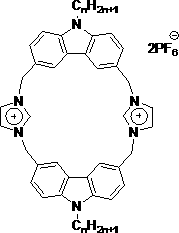
Novel carbazole-based cyclophane
A technology of carbazole and cyclopan, which is applied in the field of carbazole-based cyclopan, and achieves the effects of simple preparation method, wide application range, and wide application and promotion prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] The present invention will be further described in detail below in combination with specific embodiments.
[0013] A kind of novel fluorescent macrocyclic molecular compound containing imidazolium salt proposed according to the present invention, its preparation method is:
[0014] Add 10 mL of acetone, 5 mmol of carbazole, 7.5 mmol of NaOH, 0.008 g of TBAB to the reaction flask, reflux for 2 hours, then add 5 mmol of hexyl bromide, stir, reflux for 24 hours, cool to room temperature, and concentrate the obtained reaction solution in vacuo Finally, add a large amount of water, filter, and recrystallize from ethanol to obtain N-hexylcarbazole as a white powder with a yield of 90%;
[0015] Under nitrogen protection, add 5 mmol N-hexylcarbazole, 20 mmol paraformaldehyde, 0.5 mmol ZnCl to 20 mL of anhydrous THF 2 , stirred, added dropwise HBr solution, reacted at 60°C for 24h, and obtained 3,6-dibromomethyl-9-hexylcarbazole after post-treatment, a light yellow solid with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com