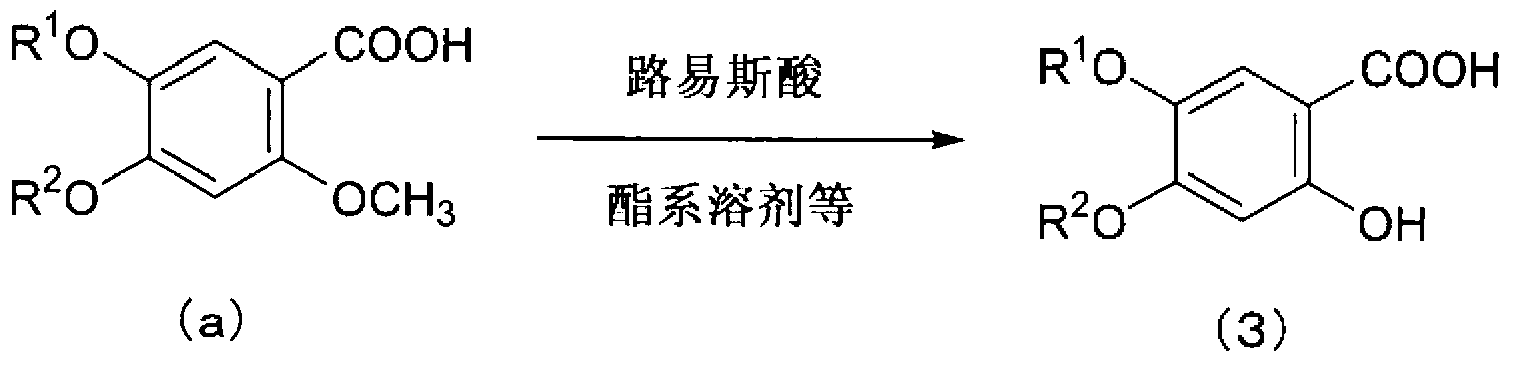
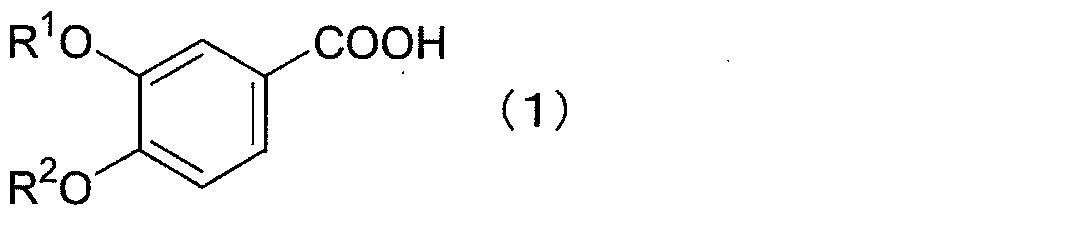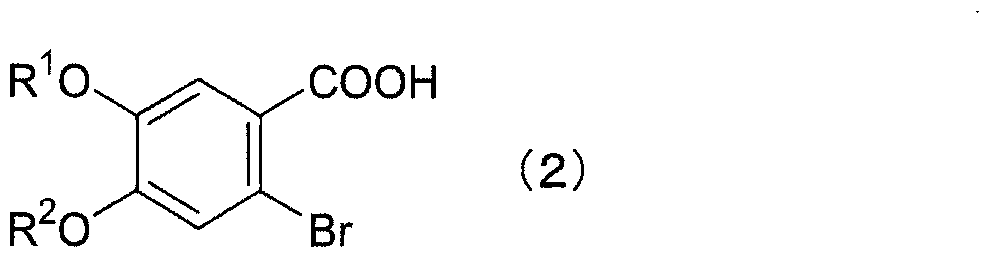Method for producing 2-bromo-4,5-dialkoxy benzoic acid
A technology of dialkoxybenzoic acid and manufacturing method, applied in the direction of carboxylate preparation, organic chemical method, chemical instrument and method, etc., can solve the problem of using a large amount of organic solvents, etc., and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (Compound (1)→Compound (2))
[0051]25.0 g of 3,4-dimethoxybenzoic acid was suspended in 500 mL of concentrated hydrochloric acid (35%), and 23.0 g (1.05 equivalent) of bromine was added dropwise at 25°C. Then, it was stirred for 7 hours. After adding 500 mL of water and stirring for 1 hour, the crystals were filtered out. After drying under reduced pressure, 34.47 g of crude crystals of 2-bromo-4,5-dimethoxybenzoic acid were obtained in a yield of 96.2%.
[0052] 1 H-NMR (DMSO-d 6 , δ): 3.79 (s, 3H), 3.84 (s, 3H), 7.21 (s, 1H), 7.37 (s, 1H), 13.08 (bs, 1H).
[0053] In the above reaction, the reaction was carried out in the same manner while changing the amount of bromine, the reaction temperature, and the reaction time. Table 1 shows the relationship between these reaction conditions and the yield. In Table 1, A represents 3-bromo-4,5-dimethoxybenzoic acid, B represents 3,4-dimethoxybenzoic acid, and C represents 1,2-dibromo-4,5-dimethoxy phenyl, E and F repres...
Embodiment 2
[0063] (Compound (2)→Compound (3))
[0064] 80 mL of water was added to 20.0 g of crude crystals of 2-bromo-4,5-dimethoxybenzoic acid obtained in Example 1 and 10.1 g of sodium carbonate. It heated and stirred to 80 degreeC, and the copper sulfate solution prepared separately from 1.91 g of copper sulfate pentahydrates, 20 mL of water, and 3.1 mL of pyridines was added. Furthermore, it heated and stirred at 90-100 degreeC for 1 hour. After cooling to 50°C, 16.0 g of concentrated hydrochloric acid was added dropwise. After cooling, the crystals were filtered out and dried under reduced pressure to obtain 15.08 g of crude crystals of 2-hydroxy-4,5-dimethoxybenzoic acid in a yield of 99.3%.
[0065] 1 H-NMR (DMSO-d 6 , δ): 3.71 (s, 3H), 3.81 (s, 3H), 6.56 (s, 1H), 7.17 (s, 1H), 11.22 (bs, 1H), 13.58 (bs, 1H).
[0066] The same reaction as in Example 2 was performed using various copper compounds equivalent to copper sulfate instead of copper sulfate. The reaction conditions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com