Replicon DNA (Deoxyribonucleic Acid) vaccine for treating chronic hepatitis B
A chronic hepatitis B and DNA vaccine technology, applied in the direction of medical preparations containing active ingredients, antibody medical ingredients, pharmaceutical formulations, etc., can solve problems such as unsuitable hepatitis B therapeutic DNA vaccines, and achieve development potential and application value. The effect of improving efficiency and enhancing immune activation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1, Construction of recombinant replicon DNA vaccine vector pSVK-HBVA and conventional DNA vaccine vector pVAX-HBVA
[0056] 1. Construction of conventional DNA vaccine plasmid pVAX-HBVA
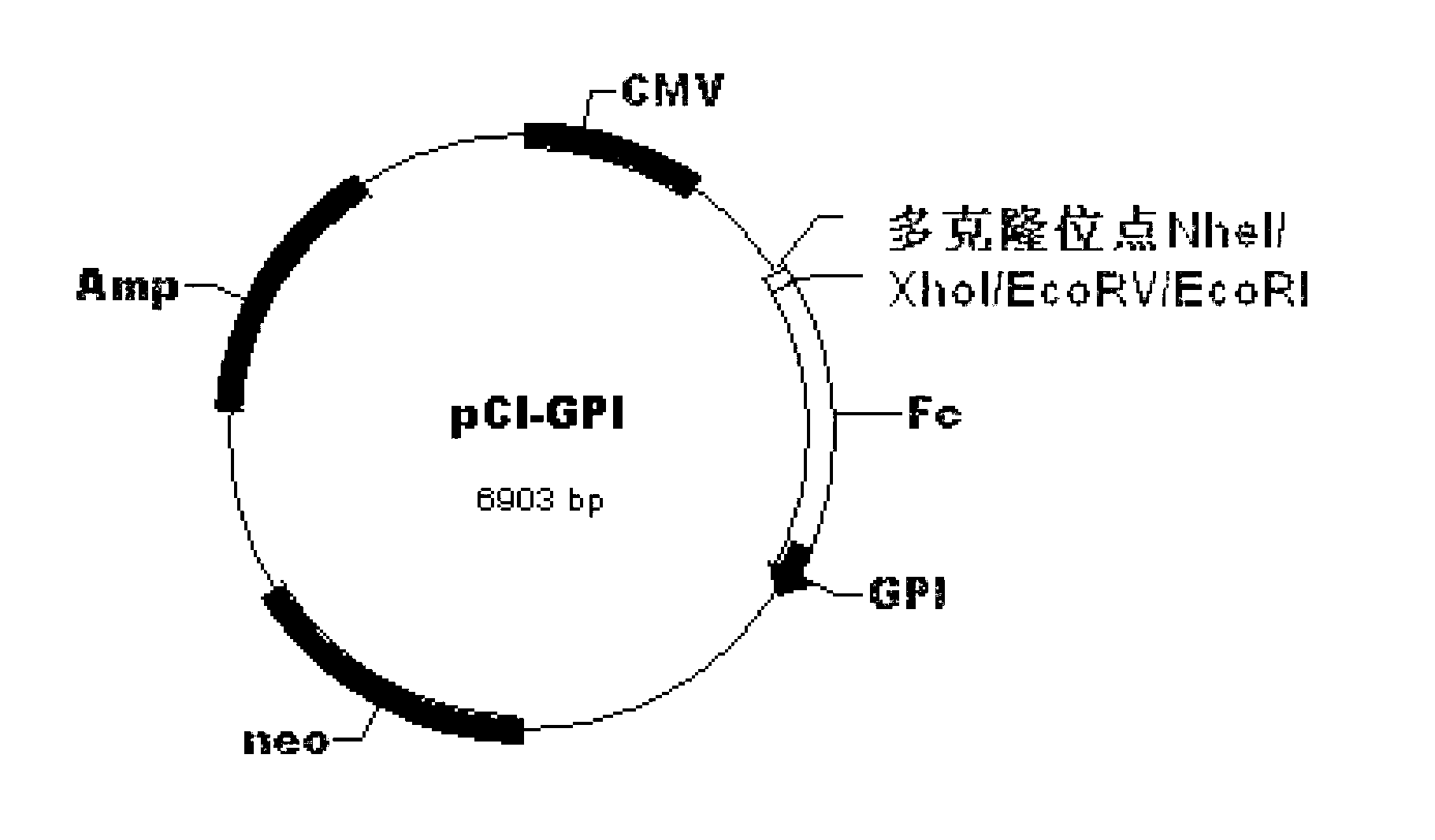
[0057] Taking pVAX1-IRES-GM / B7 (see literature: Liu Ning, Yan Jinqi, Ran Duoliang, etc. Construction and expression of pVAX1-IRES-GM / B7 immunopotentiation vector for tumor gene vaccines. Journal of the Academy of Military Medical Sciences, 2008, 32( 3):249-52) was used as the starting vector to construct a conventional DNA vaccine vector pVAX-HBVA carrying the fusion gene FL-CS, human IgG Fc gene, GPI gene, GM-CSF gene and B7.1 gene from upstream to downstream sequentially ( see Figure 9 The physical map of the recombinant plasmid pVAX-HBVA), the specific construction method includes the following steps:
[0058] 1. Design and acquisition of HBV complex antigen CS gene
[0059] Search for related sequences of HBV C, S, PreS and other genes on the NCBI website (Genebank numb...
Embodiment 2
[0098] Embodiment 2, the immunological function detection of pSVK-HBVA immune BALB / c mice
[0099] Referring to Example 1, in the process of constructing the new replicon DNA vaccine pSVK-HBVA, a conventional DNA vaccine pVAX1-HBVA based on the common plasmid vector pVAX1 was also constructed. Three dose groups of high (100 μg), medium (10 μg) and low (1 μg) of the two vaccines were set up to immunize healthy Balb / c mice, and the humoral and cellular immune responses of the mice after immunization were detected.
[0100] Immune grouping: 6-8 weeks old Balb / c female mice were randomly divided into 10 groups: ① blank group, ② normal saline group, ③ pVAX1 empty vector group, ④ pSVK empty vector group, ⑤ pVAX-HBVA1 μg, ⑥ pVAX-HBVA 10 μg , ⑦pVAX-HBVA100μg, ⑧pSVK-HBVA1μg, ⑨pSVK-HBVA10μg, ⑩pSVK-HBVA100μg, 10 animals / group.
[0101] Immunization scheme: The leg muscles of the mice were immunized according to the dosage of each group, and the vaccine was delivered by in vivo electropo...
Embodiment 3
[0112] Example 3, Cellular Immunology Detection of pSVK-HBVA Immunized HBV Tg Transgenic Mice
[0113] Example 2 shows that the new replicon DNA vaccine pSVK-HBVA can activate strong humoral and cellular immune responses in healthy Balb / c mice at a low dose of 1 μg. This example further explores the effect of the new replicon DNA vaccine pSVK-HBVA on transgenic The ability of HBV Tg (Transgenic, Tg) transgenic mice with full-length hepatitis B gene to activate immune response in vivo. HBV Tg transgenic mice (purchased from 458 Hospital) are similar to patients with chronic hepatitis B. Transgenic mice carry hepatitis B genes for a long time and are highly immune-tolerant to hepatitis B antigens. The ability to activate cellular immune responses in this model is important for the treatment of chronic hepatitis B important basis. The immunization grouping and immunization scheme formulation of the transgenic mice were the same as the immunization procedures of the normal Balb / c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com