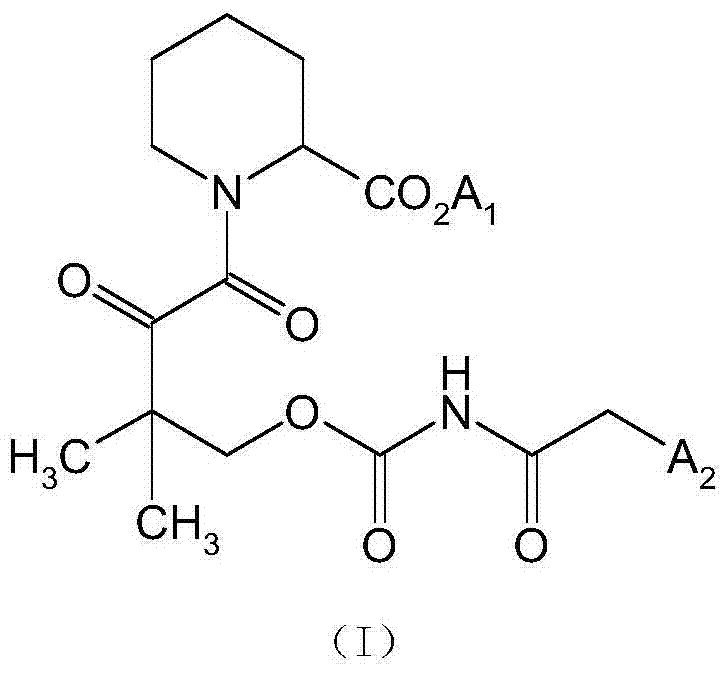
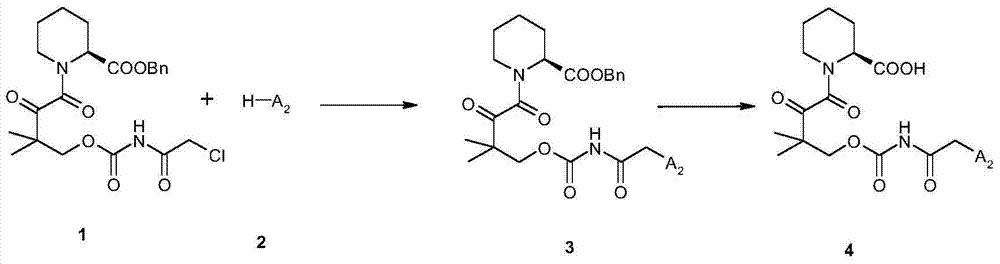
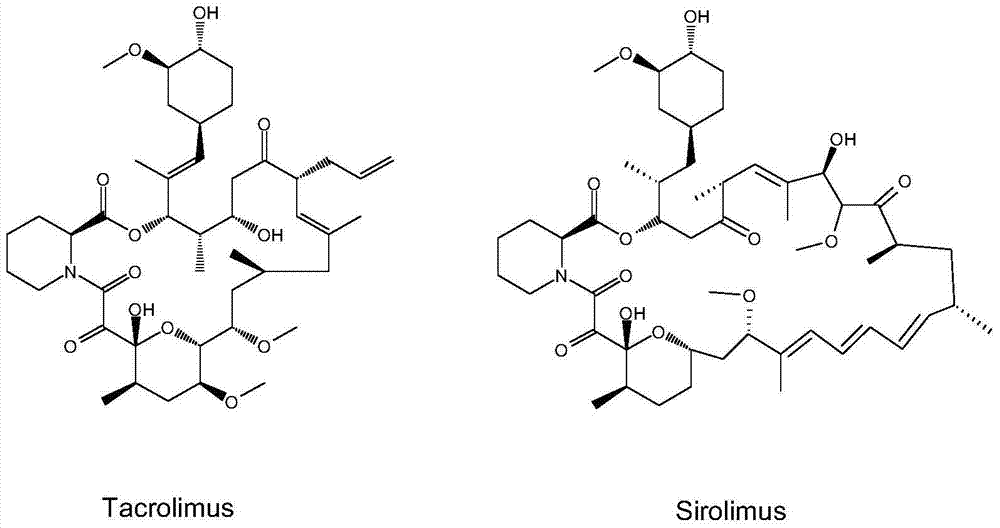
N-substituted pipecolic acid derivative, as well as preparation method and application thereof
A technology of acid derivatives and substituents, applied in the field of N-substituted pipecolic acid derivatives, which can solve the problems of weakened autophagy, reduced ability of cells to adapt to the external environment and self-defense response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0089] The general synthetic method of embodiment 3 classes:
[0090] Compound 1 (4.66 g, 10 mmol) and DMF (50 mL) were added into a 100 mL round bottom flask and stirred for 10 minutes. Add anhydrous potassium carbonate (1.66g, 12mmol) to the system, stir at room temperature for half an hour, add compound 2 (11mmol), then stir at room temperature for 2-8 hours (TLC monitors the reaction, the developer is EA / PE=2 / 1 ). After the reaction was complete, the reaction solution was diluted with ethyl acetate (500 mL), washed with water (200 mL*4), washed with saturated brine (100 mL*2), and dried over anhydrous sodium sulfate (5 g) for 2 hours. Filter off the desiccant, wash the desiccant with a small amount of ethyl acetate, pressurize the filtrate below 35°C to dry the solvent, and separate the residue with a column (the eluent is petroleum ether (PE) / ethyl acetate (EA) = 3: 1—1:1), the effective fraction was collected, concentrated to dryness under reduced pressure under 35°C t...
Embodiment 4a
[0100] Example 4a: using the general synthesis method of Example Compound 4, ethyl acetate was used as a solvent in the reaction, and the yield was 94%.
[0101] White powder. Mp.65-67℃, negative ion ESI-MS m / z: 497.6[M-H] - . 1 H-NMR (400MHz, DMSO-d6) δ: 10.349 (1H, s, 7'-NH), 4.967 (1H, d, J=4.8Hz, H-2), 4.219 (2H, m, H-4' ), 4.032 (2H, m, H-2″′), 3.403 (1H, br.d, H-6), 3.167 (1H, m, H-6), 3.348 (2H, m, H-9’) , 3.283 (4H, br.s, H-3″, 5″), 2.462 (4H, br.s, H-2″, 6″), 2.176 (1H, m, H-3), 1.596-1.702 ( 3H, m, H-3, 4), 1.297-1.348 (2H, m, H-5), 1.254 (3H, s, H-CH 3 ), 1.241 (3H, s, H-CH 3 ), 1.168(3H,t,J 1,2 =J 2,3 =6.8Hz, H-3"').
[0102] 4b: Using the general synthesis method of Example Compound 4, the reaction solvent is ethyl acetate, and the yield is 92%.
[0103]
[0104] White powder. Mp.146-148℃, positive ion ESI-MS m / z: 503.5[M+H] + . 1 H-NMR (400MHz, DMSO-d6) δ: 10.345 (1H, s, 7'-NH), 7.200 (2H, t, J 1,2 =8.4Hz,J 2,3 =7.6Hz, H-3"', 5"'), 6.926(2H,d,J...
preparation Embodiment 1
[0205] tablet:
[0206] Preparation method: mix Example compound 4K, lactose and cornstarch, moisten evenly with water, sieve and dry, then sieve, add magnesium stearate, and then press the mixture into tablets, each weighing 250 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com