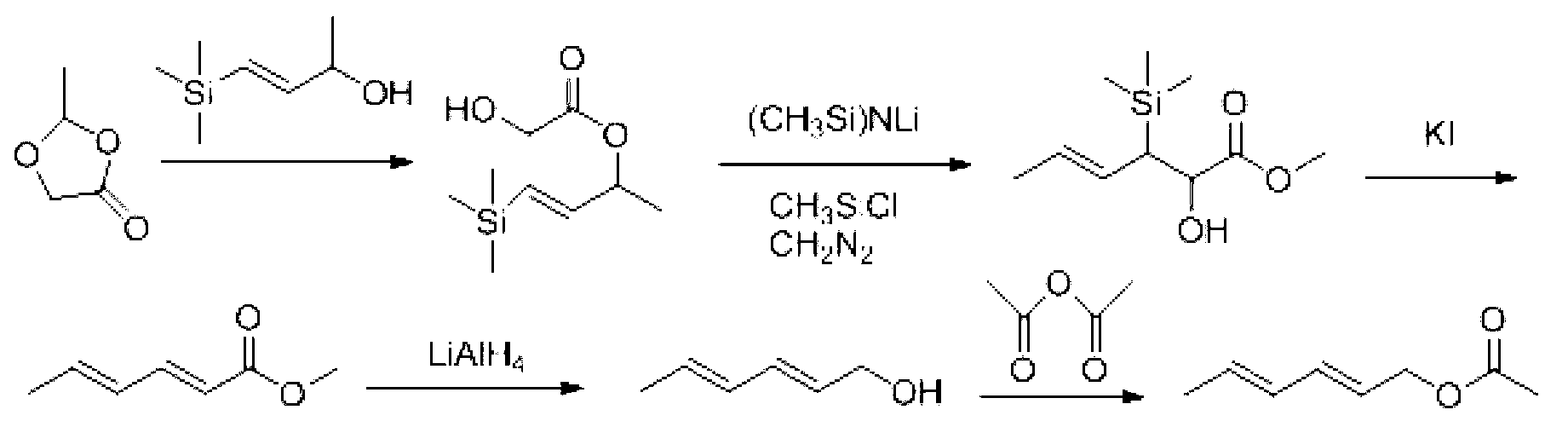
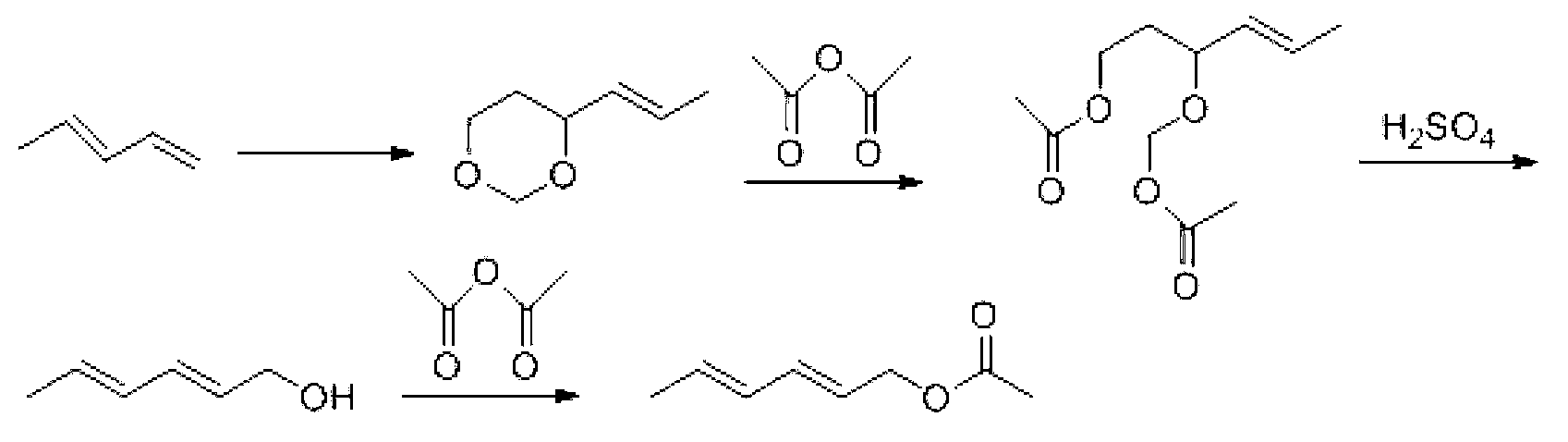
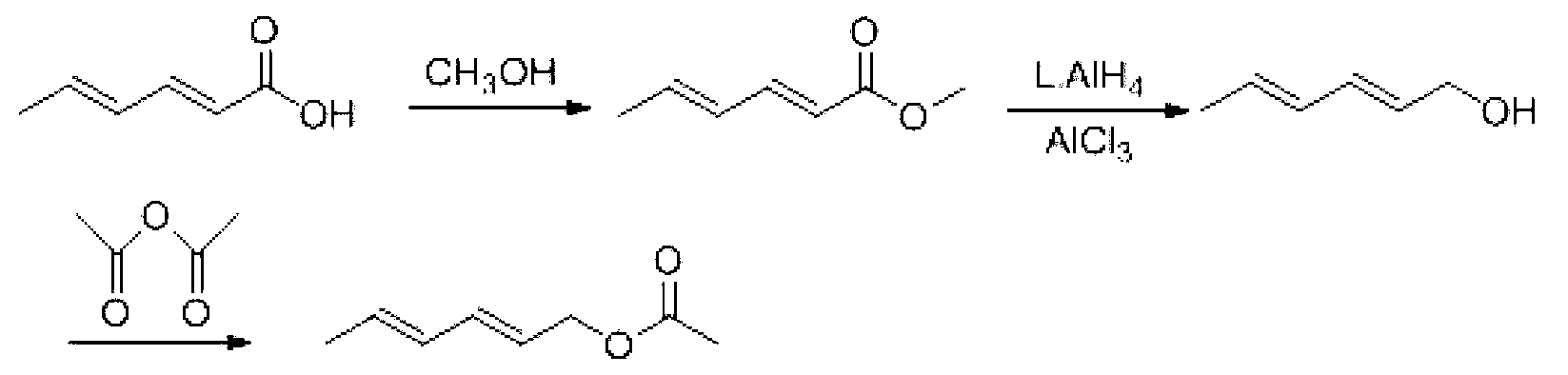
Preparation method for trans, trans-2,4-hexadiene acetate
A technology of hexadienal acetate and hexadienol, which is applied to the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., can solve problems such as dangerous processes, difficult industrial production, and high costs. Achieve the effects of high reaction safety, easy industrial production, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Trans, the preparation method of trans-2,4-hexadienal acetate, the steps are as follows:
[0040] ①In a 500mL three-necked flask equipped with a thermometer, mechanical stirring and a constant pressure dropping funnel, add 33.6g of sorbic acid (0.3mol), 30.3g of triethylamine (0.3mol), 340g of anhydrous THF, and protect with nitrogen. Cool in an ice bath to obtain a colorless transparent liquid. Add 32.55 g (0.3 mol) of ethyl chloroformate dropwise at 0°C, keeping the temperature below 10°C. After dripping, continue to react in ice-salt bath for 2h. Gas phase monitoring, stop the reaction after the reactants are completely consumed. Quickly filter to remove triethylamine hydrochloride insoluble matter, and the filtrate is a yellow clear liquid. The solvent THF was evaporated to dryness under reduced pressure at 45°C to obtain 54.71 g of an orange liquid with a yield of 99% and a GC normalized purity of 96%.
[0041] ②In a 1L three-necked flask equipped with a mechan...
Embodiment 2
[0049] Trans, the preparation method of trans-2,4-hexadienal acetate, the steps are as follows:
[0050] ①In a 500mL three-necked flask equipped with a thermometer, mechanical stirring and a constant pressure dropping funnel, add 44.8g of sorbic acid (0.4mol), 40.4g of triethylamine (0.4mol), 450g of anhydrous ether, and protect it under nitrogen. Cool in an ice bath to obtain a colorless transparent liquid. Add 43.40 g (0.4 mol) of ethyl chloroformate dropwise at 0°C, keeping the temperature below 10°C. After dripping, continue to react in ice-salt bath for 3h. Gas phase monitoring, stop the reaction after the reactants are completely consumed. Quickly filter to remove triethylamine hydrochloride insoluble matter, and the filtrate is a yellow clear liquid. The diethyl ether was evaporated to dryness under reduced pressure at 45°C to obtain 72.94 g of an orange liquid with a yield of 99% and a GC normalized purity of 96%.
[0051] ②In a 1L three-necked flask equipped with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com