Novel 18f-labeled substituted quinazoline compounds and their preparation methods and tumor pet imaging applications
A technology of quinazolines and 18F, which is applied in the field of new 18F-labeled substituted quinazolines to achieve high labeling rate, short labeling time, and high tumor uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
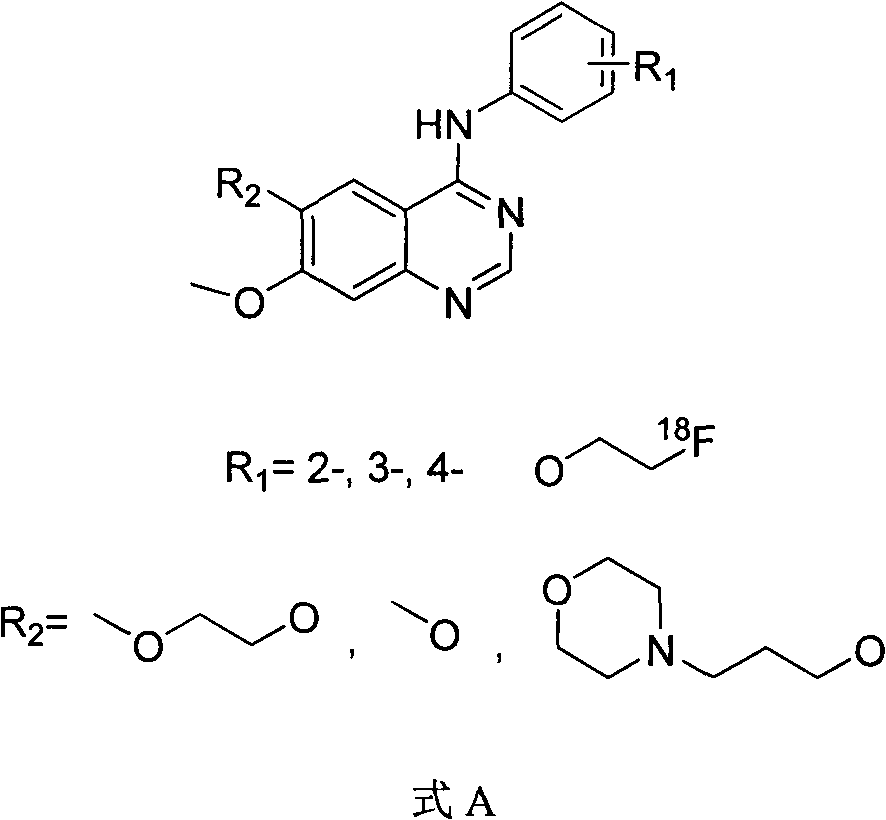
[0066] Embodiment formula G: in formula A, R1 is in para-position, R2=methoxyl group ([ 18 F]-FEWHIP131)
[0067]
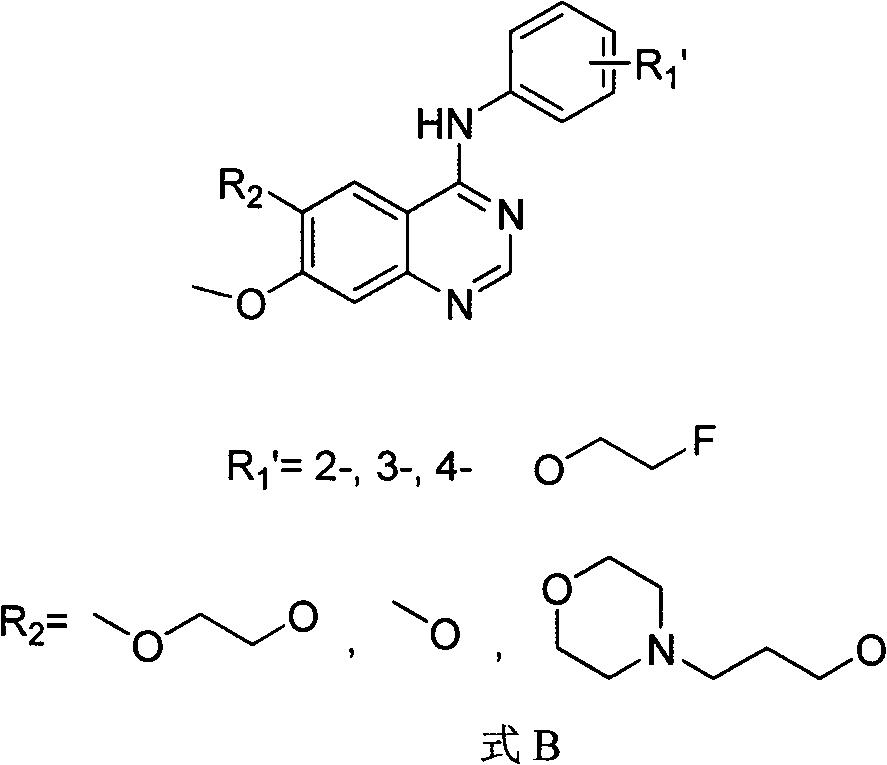
[0068] One, prepared according to the following steps is that R1 is in the para-position in formula A, and the compound of R2=methoxyl group ([ 18 F]-FEWHIP131), including the synthesis of its labeling precursor (R1' is in the para position in formula B, R2 = methoxy) and the synthesis of the labeling precursor 19 Synthesis of F Substitutes and Radiochemical Synthesis in Three Parts
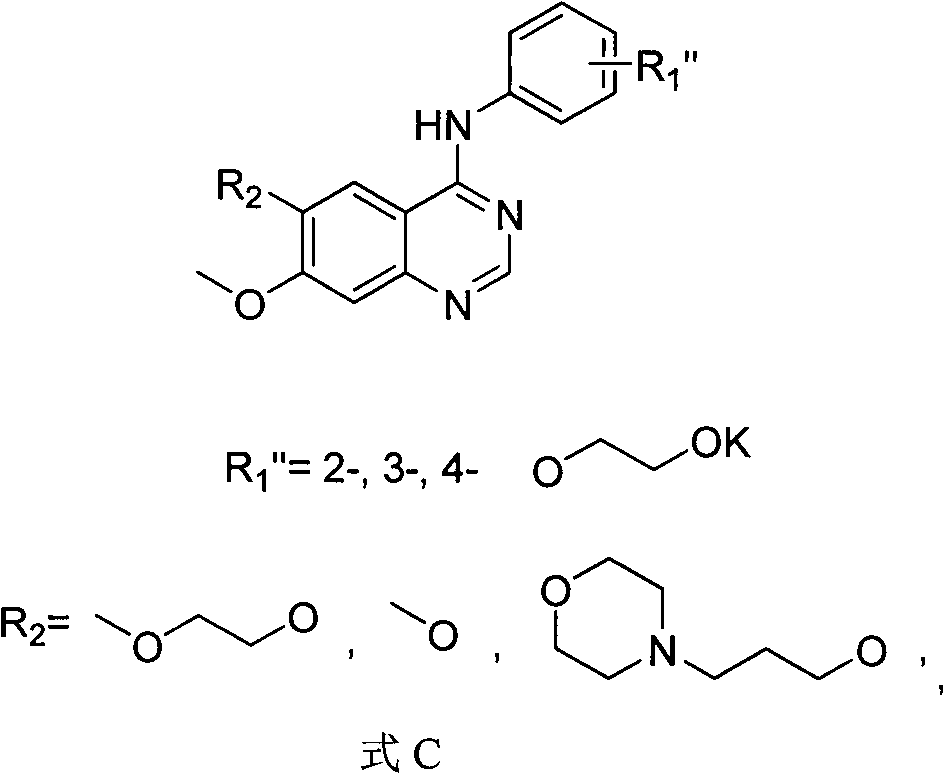
[0069] 1. Synthesis of labeled precursors
[0070] According to the following steps, the compound in which R1" is in the para position and R2 = methoxy group in formula C is prepared according to the following steps
[0071] 1.13, Synthesis of ethyl 4-dimethoxybenzoate
[0072] 3,4-Dimethoxybenzoic acid (10g, 0.055mol) and ethanol (100mL) were added into a 250mL reaction flask, and concentrated H2SO4 (8mL) was added dropwise under stirring, heated to reflux, followed by TLC m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com