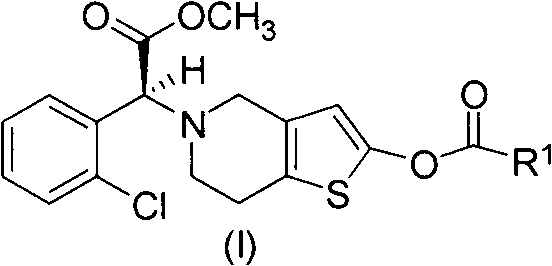
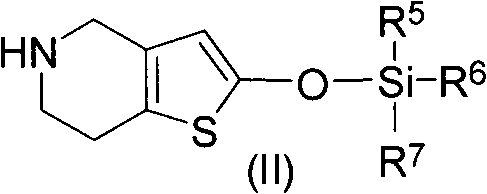
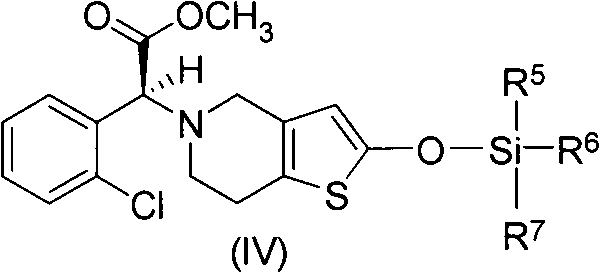
Method for preparing vicagrel and derivatives thereof
A compound, phenyl technology, applied in the field of preparation of vicagrel and its derivatives, can solve the problems of carcinogenicity, poor product optical purity and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 2-tert-butyldimethylsiloxotetrahydrothieno[3,2-c]pyridine
[0029]Dissolve 3g (0.0157mol) of 5,6,7,7a-tetrahydrothieno[3.2-c]pyridin-2(4H)-one hydrochloride in 60mL of acetonitrile, add 2mL of triethylamine, stir at 20oC until solution clarify. Then add 2.2g (0.0146mol) tert-butyldimethylsilyl chloride, keep the temperature constant, and stir for 6h. The disappearance of raw materials was detected by TLC, the insoluble matter was filtered, and the filtrate was evaporated to dryness to obtain a brown sticky substance, namely 2-tert-butyldimethylsiloxotetrahydrothieno[3,2-c]pyridine: 3.8g, yield 90 %.
[0030] 1H-NMR (300MHz, CDCl3) δ0.22(s, 6H), 0.96(s, 9H), 2.65(m, 2H), 3.17(m, 2H), 3.77(s, 2H), 5.29(s, 1H ), 5.76(s, 1H); ESI-MS m / z 270.1[M+H]+.
Embodiment 2
[0032] (S)-2-(2-((tert-butyldimethylsilyl)oxy)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2 -(2-Chlorophenyl)-methyl acetate
[0033] 500mg (1.3mmol) (R)-2-(2-chlorophenyl)-2-(4-nitrobenzenesulfonyloxy)-acetic acid methyl ester, 395mg (1.5mmol) 2-tert-butyldimethyl Siloxotetrahydrothieno[3,2-c]pyridine and 328 mg (2.5 mmol) of potassium bicarbonate were added to 10 mL of acetonitrile, the reaction system was protected with nitrogen, and stirred overnight at 25°C. After the reaction solution was allowed to stand, the insoluble matter was filtered to obtain a yellow mother liquor. The solvent was evaporated to dryness under reduced pressure, and a colorless oil was obtained by flash column chromatography (petroleum ether: ethyl acetate = 50:1), namely (S)-2-(2-((tert-butyldimethylsilyl) Oxy)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetic acid methyl ester: 500 mg, yield 85%. 1 H-NMR (300MHz, CDCl 3 )δ0.24(s, 6H), 0.95(s, 9H), 2.69-2.85(m, 4H), 3.43-3.56(m...
Embodiment 3
[0035] (S)-2-(2-((tert-butyldimethylsilyl)oxy)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2 -(2-Chlorophenyl)-methyl acetate
[0036] 250mg (0.65mmol) (R)-2-(2-chlorophenyl)-2-(4-nitrobenzenesulfonyloxy)-acetic acid methyl ester, 175mg (0.65mmol) 2-tert-butyldimethyl Siloxotetrahydrothieno[3,2-c]pyridine and 0.09 mL (0.65 mmol) of triethylamine were added to 5 mL of acetonitrile, the reaction system was protected with nitrogen, and stirred overnight at 25°C. After the reaction solution was allowed to stand, the insoluble matter was filtered to obtain a yellow mother liquor. The solvent was evaporated to dryness under reduced pressure, and a colorless oil was obtained by flash column chromatography (petroleum ether: ethyl acetate = 50:1), namely (S)-2-(2-((tert-butyldimethylsilyl) Oxy)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetic acid methyl ester: 220 mg, yield 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com