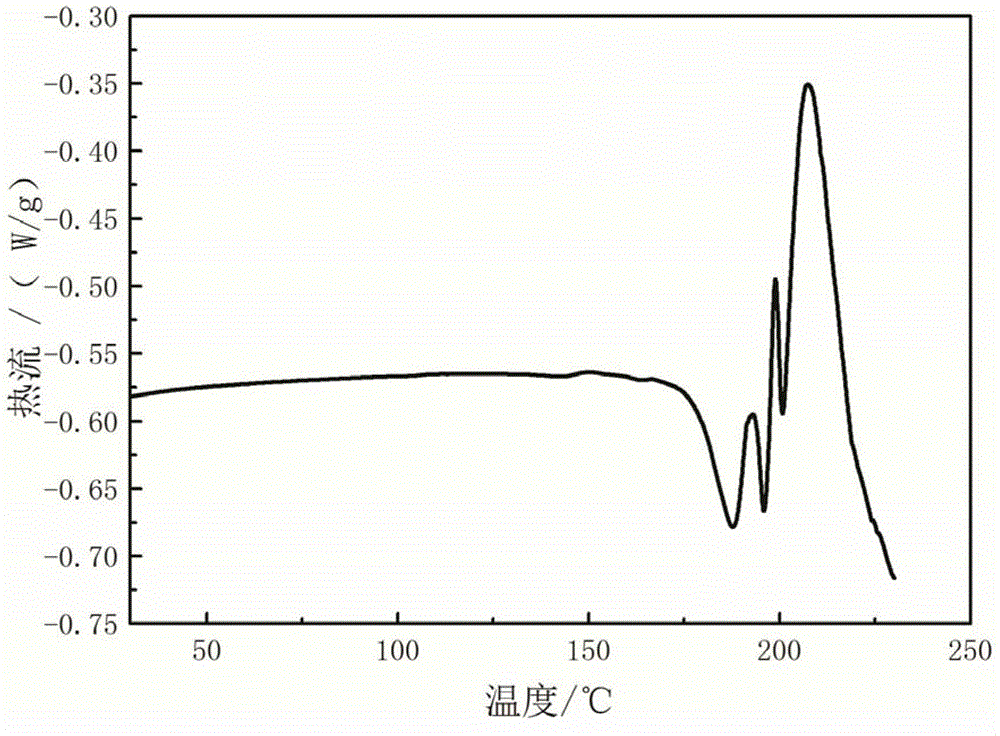
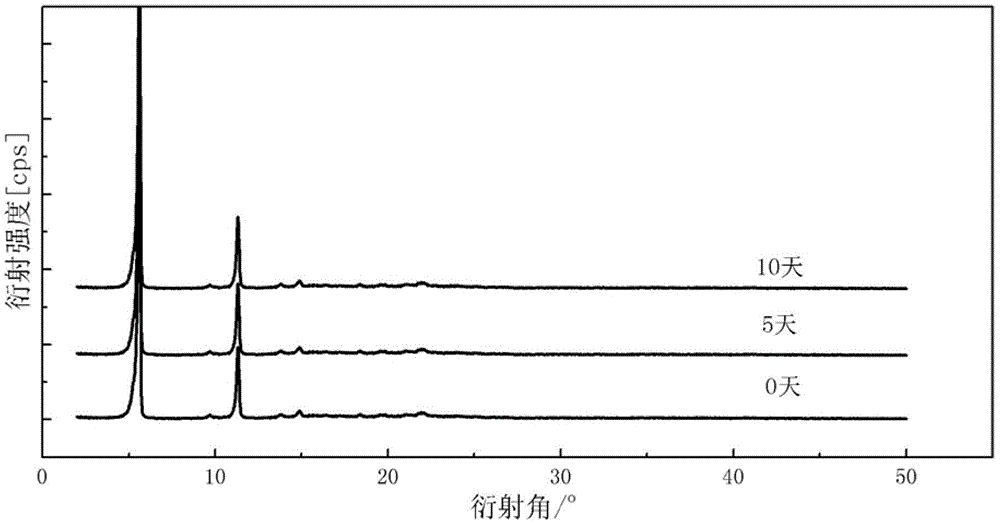
New crystal form α of clindamycin phosphate and its preparation method
A technology of clindamycin phosphate and crystal form, which is applied in the field of medicine and can solve the problems of poor stability and unstable dissolution of clindamycin phosphate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of Clindamycin Phosphate Crystal Form α
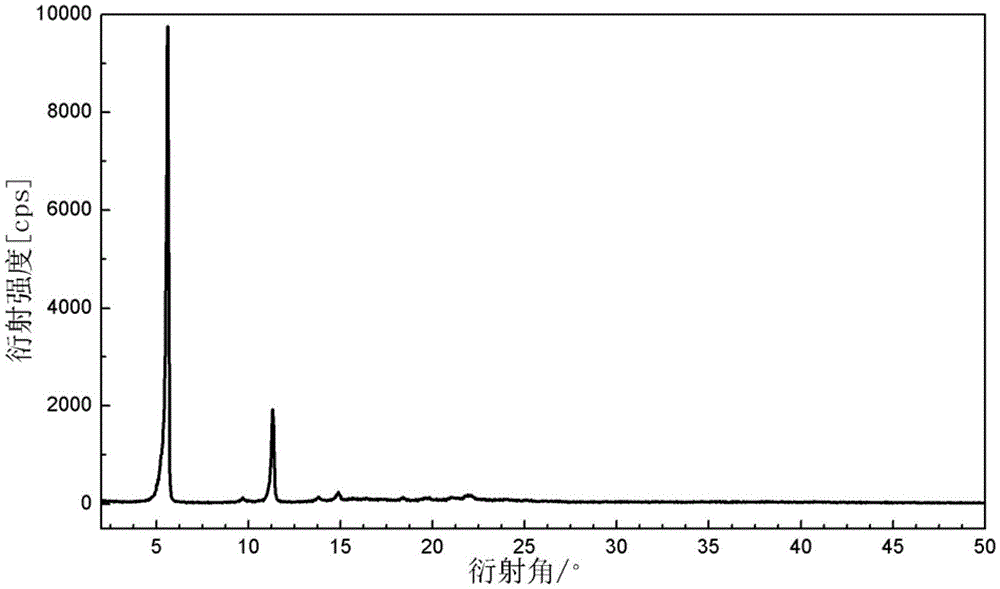
[0023] Dissolve 5g of crude lindamycin phosphate in a mixed solvent of 20mL ethanol and 20mL water, then heat up to 65°C and dissolve, heat filter to remove insoluble impurities, stir, cool down to 35-37°C to produce crystals, and keep warm for 2- After 3 hours, the temperature was lowered to 5°C, and dried at 50°C for 24 hours to obtain the crystal form α of clindamycin phosphate. The powder X-ray diffraction pattern of the product and figure 1 unanimous.
Embodiment 2
[0024] Example 2 Preparation of Clindamycin Phosphate Crystal Form α
[0025] Dissolve 4g of crude lindamycin phosphate in a mixed solvent of 20mL ethanol and 10mL water, then heat up to 50°C and dissolve, heat filter to remove insoluble impurities, stir, cool to 30-32°C to produce crystals, and keep warm for 2- After 3 hours, the temperature was lowered to 5°C, and dried at 55°C for 24 hours to obtain the crystal form α of clindamycin phosphate. The powder X-ray diffraction pattern of the product and figure 1 unanimous.
Embodiment 3
[0026] Example 3 Preparation of Clindamycin Phosphate Crystal Form α
[0027] Dissolve 4.5g of crude lindamycin phosphate in a mixed solvent of 30mL of ethanol and 6mL of water, then heat up to 60°C and dissolve, heat filter to remove insoluble impurities, stir, cool to 45-47°C to produce crystals, and keep warm for 2 -3h, then lower the temperature to 5°C, and dry at 60°C for 24 hours to obtain the crystal form α of clindamycin phosphate. The powder X-ray diffraction pattern of the product and figure 1 unanimous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com