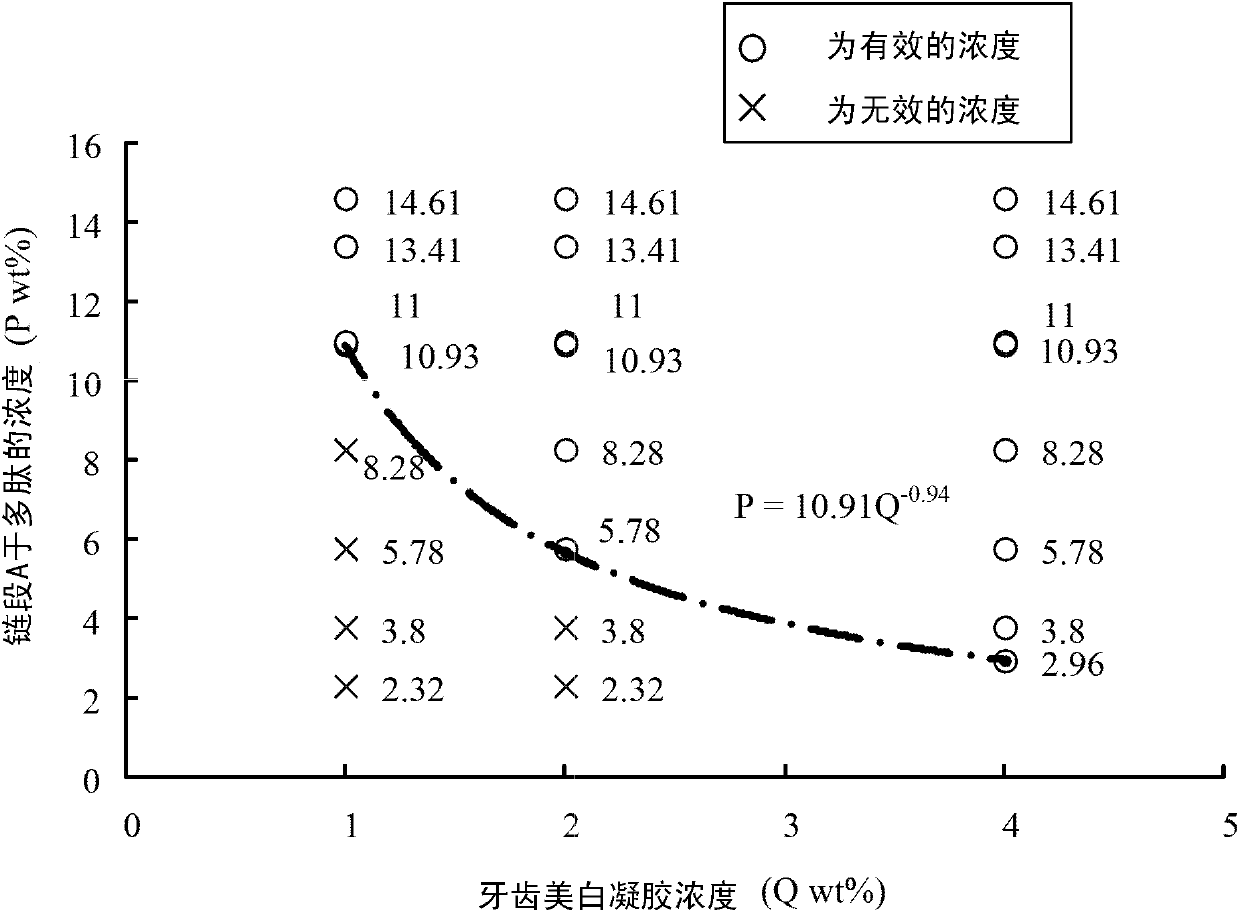
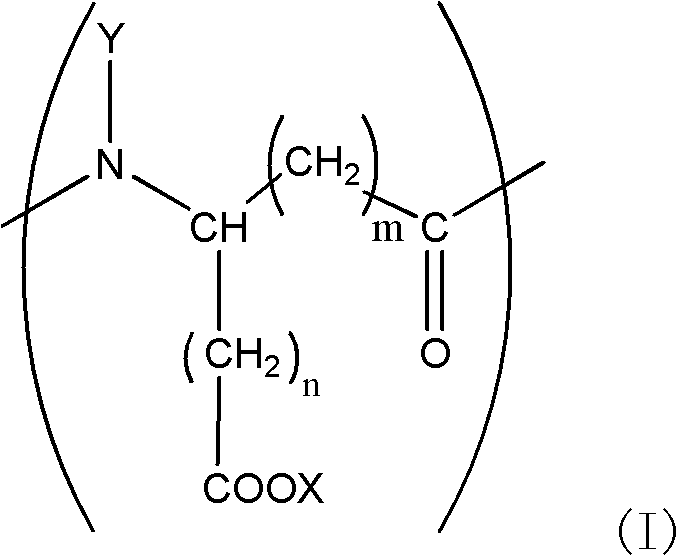
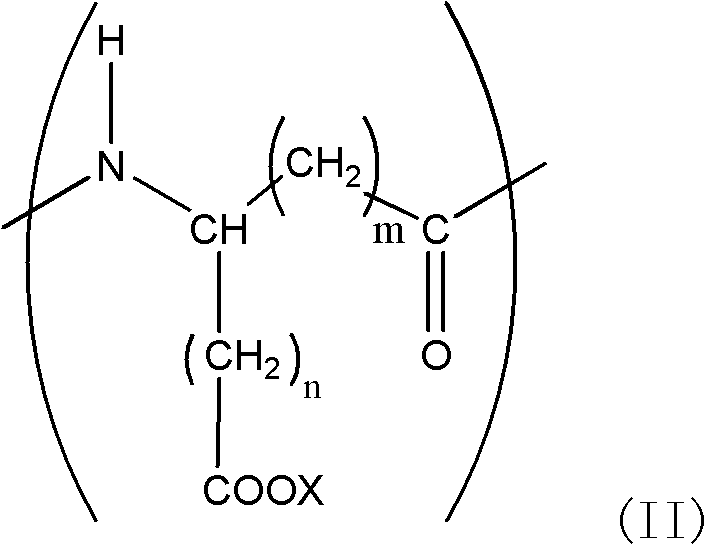
Tooth whitening gel
A technology for teeth whitening and whitening gel, which is used in dentistry, medical science, oral care and other directions to achieve the effects of optimal stability and safety, high biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Preparation method of tooth whitening gel and halogenated polypeptide
[0038]The teeth whitening gel provided by the embodiments of the present invention is prepared by diluting or dispersing the halogenated polypeptide indicated in the present invention with water to prepare tooth whitening gels with different concentrations.
[0039] To prepare the halogenated polypeptide required by the embodiments of the present invention, polyglutamic acid and sodium hypochlorite can be used for chlorination reaction at room temperature, and an aqueous solution containing phosphoric acid is used as a buffer solution to adjust its pH to 6-8. After the preset reaction time is completed, an appropriate amount of isopropanol is added to precipitate the product. Then filter and vacuum dry to obtain chlorinated polyglutamic acid (that is, a kind of halogenated polypeptide pointed out in the present invention), which is white to light yellow in powder form.
[0040] The polyglutamic aci...
Embodiment 1
[0058] Take 10.0 g of polyglutamic acid (polyglutamic acid sodium salt, molecular weight about 2,000,000 Daltons, Vedan, Taiwan) into a 250 ml single-necked bottle, add 90 ml of pure water, and stir until completely dissolved. Then add 4 grams of 12.65 wt% sodium hypochlorite aqueous solution, and then adjust the pH value of the reaction solution to 6-8 with 0.5N phosphoric acid aqueous solution. Stirring of the reaction was continued at room temperature for 30 minutes. The reaction solution was placed in a separatory funnel, chlorinated polyglutamic acid 1 was precipitated with 400 ml of isopropanol, and separated from the separatory funnel. Next, the chlorinated polyglutamic acid 1 was dried under vacuum. Chlorinated polyglutamic acid 1 after drying is white to pale yellow in powder form and can be completely dissolved in aqueous solution.
[0059] Titrate the dried chlorinated polyglutamic acid 1 with sodium thiosulfate aqueous solution, and finally obtain the concentrati...
Embodiment 2~7
[0061] Embodiment The reaction conditions are as described in Example 1, but the weight of the above-mentioned 12.65wt% sodium hypochlorite aqueous solution is increased to 7.5, 12, 20, 28, 36 and 40 grams respectively, and added to the reaction solution. Stirring of the reaction was continued at room temperature for 30 minutes. The reaction solutions of each group were respectively placed in a separatory funnel, chlorinated polyglutamic acid 2-7 was precipitated with 400 ml of isopropanol, and separated from the separatory funnel. Next, dry the chlorinated polyglutamic acid 2-7 under vacuum. The dried chlorinated polyglutamic acid 2-7 is white to pale yellow in powder form and can be completely dissolved in aqueous solution.
[0062] Titrate the dried chlorinated polyglutamic acid 2-7 with sodium thiosulfate aqueous solution respectively, and finally obtain the segment A with N-Cl functional group in chlorinated polyglutamic acid 2-7. concentration in amino acid (see Table ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com