Hydroxylamine synthesis method
A synthesis method, hydroxylamine technology, applied in organic chemistry methods, chemical instruments and methods, and the formation/introduction of functional groups, etc., can solve the problems of cost, toxicity, and purification that is not suitable for high volume, increase project cost, and difficult to purify.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
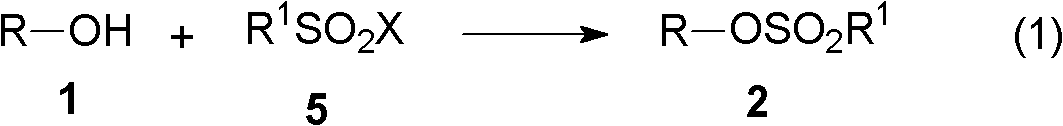
[0072] The present invention relates to the synthetic method of hydroxylamine, comprises the following steps:
[0073] (A) First, in the presence of an acid-binding agent, the alcohol reacts with an alkylsulfonyl halide to generate a sulfonate;
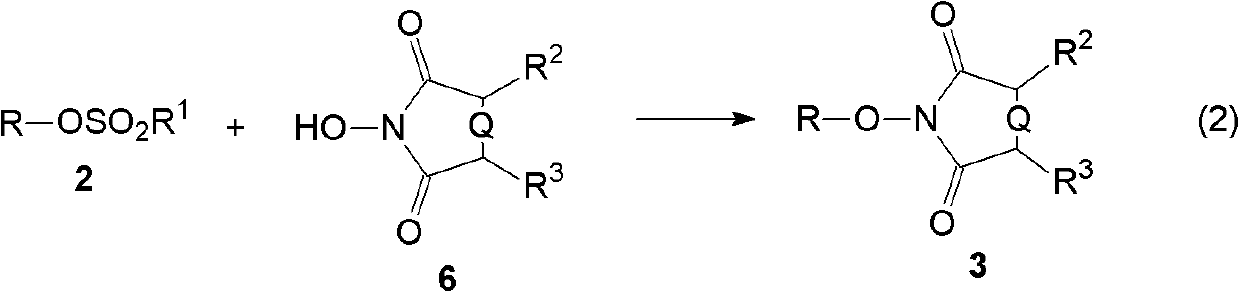
[0074] (B) then reacting the sulfonic acid ester obtained in step (A) with N-hydroxyl cyclic imide in the presence of a base to generate an alkylated product of N-hydroxyl cyclic imide;
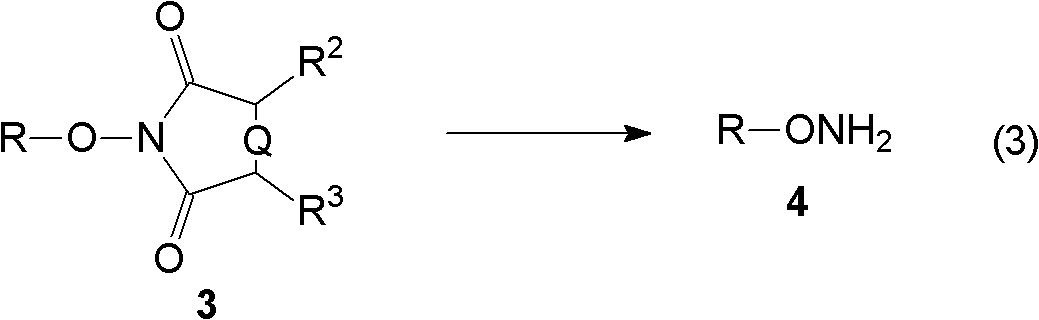
[0075] (C) reacting the alkylated product obtained in step (B) with an aminolysis reagent or a hydrazinolysis reagent to obtain hydroxylamine.
[0076] In a preferred embodiment, the present invention relates to a synthetic method of hydroxylamine, comprising the following steps:
[0077] (A) Step 1, in the presence of an acid-binding agent, the alcohol represented by Chemical Formula 1 reacts with the alkylsulfonyl halide of Chemical Formula 5 to generate a sulfonate represented by Chemical Formula 2,
[0078]
[0079] (B) step 2, in the presen...
Embodiment 1
[0149]
[0150] step 1:
[0151] Add raw material 1a (47g, 0.4mol, 1eq), triethylamine (51.8g, 0.51mol, 1.28eq), MTBE (400ml) into a 1L reaction flask, drop MsCl (50.4g , 0.44mol, 1.1eq). After dropping, stir at room temperature for 2 hours to stop the reaction. The organic phase was sequentially washed with saturated NaHCO 3 washed, washed with saturated brine, dried and concentrated to obtain the product (83.3 g, 0.4 mol) with a yield of 100%.
[0152] 1 H NMR (CDCl 3 ): 4.33(t, 2H); 3.63(t, 2H); 3.06(s, 3H); 1.20(s, 9H).
[0153] Step 2:
[0154] N-hydroxyphthalimide (3.46g, 0.021mol, 1eq), 2a (5g, 0.025mol, 1.2eq), sodium bicarbonate (2.139g, 0.025mol, 1.2eq), DMF (15ml) were added to In a 50ml reaction bottle, set the temperature at 80°C and react for 24 hours. The temperature was lowered, and the ice-water mixture (10V) was poured into the reaction solution. A solid was precipitated, and the solid was filtered, washed and dried to obtain the product (3.5g0.01...
Embodiment 2
[0160]
[0161] step 1:
[0162] The preparation method is the same as 2a, and the yield is 96%.
[0163] LC-MS: 155.4 (M + ).
[0164] 1 H NMR (CDCl 3 ): 4.23(t, 2H), 3.03(s, 3H), 1.75(m, 2H), 1.45(m, 2H), 0.95(t, 3H).
[0165] Step 2:
[0166] The preparation method is the same as 3a, and the yield is 65.9%.
[0167] LC-MS: 220.46 (M+1).
[0168] 1 H NMR: (CDCl 3 ): 7.83 (d, 2H); 7.76 (d, 2H); 4.25 (d, 2H); 1.77 (m, 2H); 1.50 (m, 2H); 0.98 (m, 3H).
[0169] Step 3:
[0170] The preparation method is the same as 4a, release the pressure, add EA / HCl solution directly after filtration to form a salt. The resulting solid was filtered and the product yield was 93%. 1 H NMR (D 2 O): 4.0 (t, 3H); 1.64 (t, 3H); 1.37 (m, 3H); 0.90 (t, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com