2-fluoro-aniline quinazoline tumour positron imaging agents as well as preparation method and application thereof
A technology for substituting aniline quinazoline and aniline quinazoline, which is applied in the fields of organic chemistry and radioactive carriers, can solve the problems of false positive, false negative, and inability to distinguish inflammation and tumor, etc., and achieves the goal of simple labeling method and automatic production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 2- 18 Radiochemical Synthesis of F-6,7-Dimethoxyquinazoline-4-aniline
[0033] see figure 1 , using a cyclotron to apply bombardment 18 O water, through 18 O(p n) 18 F nuclear reaction produces 100mCi 18 F ions, which will be accelerator-produced 18 F is captured by the QMA column;
[0034] ⑴ Use 1mL solution (12.5 mg K 222 Add 2.5mg K 2 CO 3 Dissolved in 0.1mL water and 0.9mL acetonitrile mixed solution) will 18 F is rinsed into the reaction tube;
[0035] (2) Dry the solution in the reaction tube at 116°C with nitrogen;
[0036] (3) Add 2 mL of dry acetonitrile in No. 2 bottle to the reaction tube, and blow dry at 116°C with nitrogen;
[0037] (4) Add 5 mg of 2-chloro-6,7-dimethoxyquinazoline-4-aniline precursor and 0.8 mL of dry DMSO in bottle No. 3 to the reaction tube, and react at 140 ° C for 10 min;
[0038]⑸ Add 30mL of water from No. 5 bottle to the reaction tube three times, pass the mixed liquid through the C-18 column between the V7 an...
Embodiment 2
[0040] Example 2 2- 18 Radiochemical Synthesis of F-6,7-Dimethoxyquinazoline-4-aniline
[0041] Applied Cyclotron Applied Bombardment 18 O water, through 18 O(p n) 18 F nuclear reaction produces 100mCi 18 F ions, which will be accelerator-produced 18 F is captured by the QMA column;
[0042] ⑴ Use 1mL solution (12.5 mg K 222 Add 2.5mg K 2 CO 3 Dissolved in 0.1mL water and 0.9mL acetonitrile mixed solution) will 18 F is rinsed into the reaction tube;
[0043] (2) Dry the solution in the reaction tube at 116°C with nitrogen;
[0044] (3) Add 2 mL of dry acetonitrile in No. 2 bottle to the reaction tube, and blow dry at 116°C with nitrogen;
[0045] (4) Add 5 mg of 2-bromo-6,7-dimethoxyquinazoline-4-N-tert-butylcarbonyl-aniline and 0.8 mL of dry DMF in bottle No. 3 to the reaction tube, and react at 140 °C for 10 min;
[0046] ⑸Add 2mL hydrochloric acid solution in No. 6 bottle to the reaction tube and hydrolyze for 15min
[0047] ⑹Add 30mL water to the No. 5 bottle ...
Embodiment 3
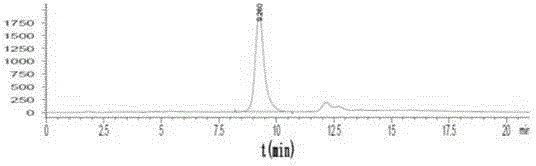
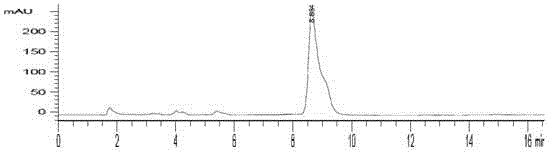
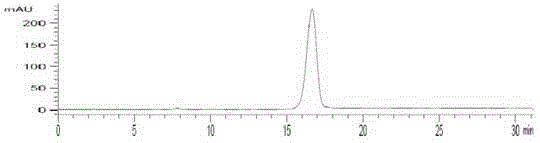
[0049] Example 3 2- 18 Quality control of F-6,7-dimethoxyquinazoline-4-aniline
[0050] See HPLC chromatogram figure 2 , 3. The quality control conditions are 50% acetonitrile aqueous solution, C-18 (4.5mm×250mm) column, ultraviolet 254nm and radioactive detector. The results show that the standard product has the same ultraviolet absorption and radioactive absorption. It is the same substance, and the synthesis time is 45min. , uncorrected synthetic yield 20–30%, radiochemical purity >98%. The spectrum of the standard product is: ESI-MS(m / z,%):300([M+H] + ,100), 1 H NMR(CDCl3,400MHz): 7.69(2H,d, J =7.93,4-position benzene ring 2’-H,6’-H); 7.45(1H,br.s,-NH); 7.42 (2H,dd, J 1 =7.53Hz, J 2 =8.32Hz, 4-position benzene ring 3’-H,5’-H); 7.20 (1H,dd, J 1 =7.14Hz, J 2 =6.34Hz,4-position benzene ring 4’-H); 7.15(1H,s,8-H); 7.04(1H,s,5-H); 4.00 (6H,s,2-OCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com