Preparation method of crizotinib intermediate (1S)-1-(2, 6-dichloro-3-fluorophenyl)ethanol
A technology of fluorophenyl and ethanol, which is applied in the field of preparation of crizotinib intermediate-1-ethanol, can solve the problems of unfavorable large-scale production, many steps, low yield, etc., and achieve high industrial application value, solvent The effect of less consumption and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
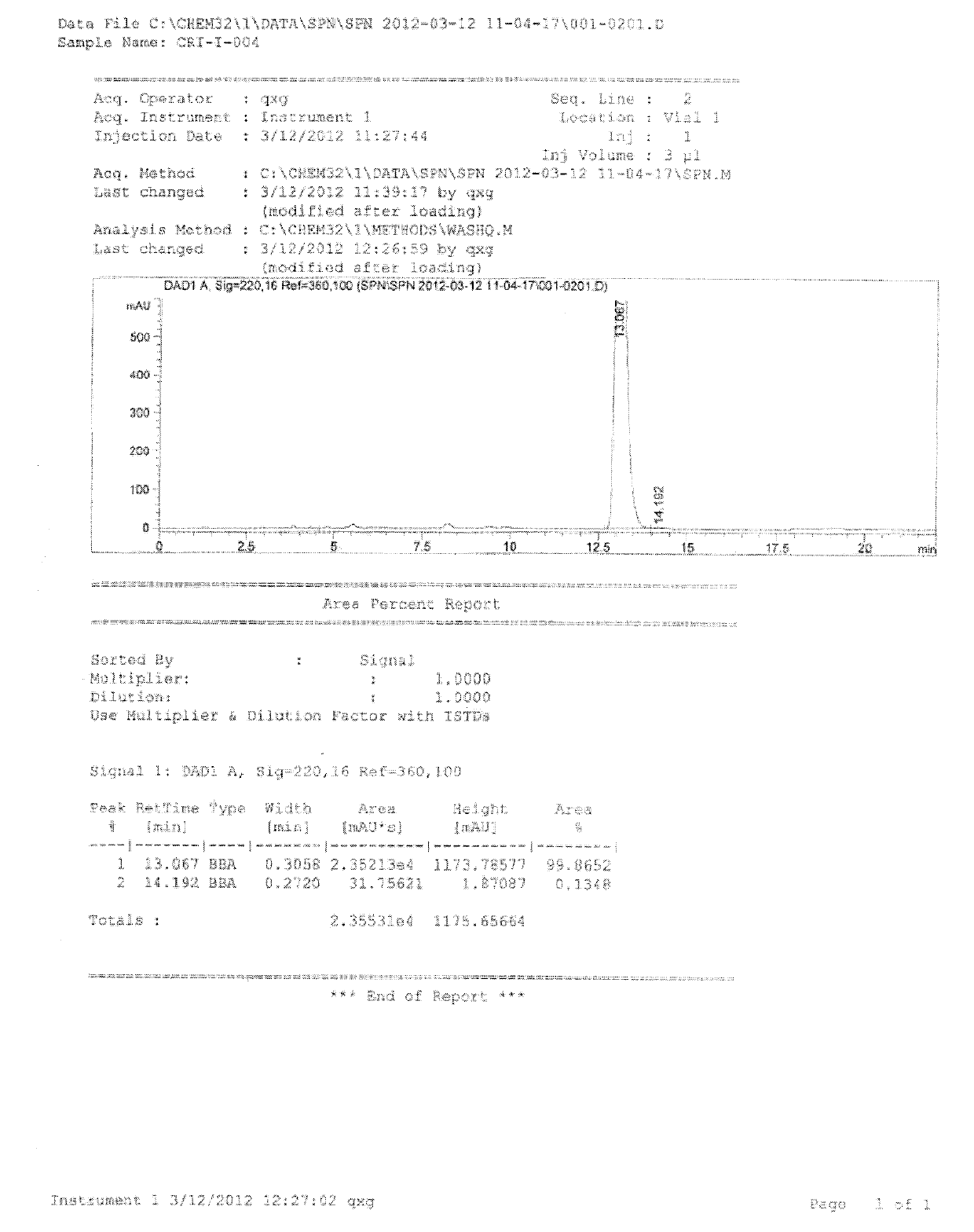
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of (1S)-1-(2,6-dichloro-3-fluorophenyl)ethanol
[0038]Under the protection of nitrogen, weigh 1.0mg (0.001mmol) chiral catalyst I (X is hydrogen) and 48mg (0.5mmol) sodium tert-butoxide to the reaction inner tube, add 3mL ethanol and 1035mg (5mmol) sodium tert-butoxide to the reaction inner tube ) 1-(2,6-dichloro-3-fluorophenyl)ethanone, put the reaction inner tube into the autoclave, replace the gas in the autoclave with hydrogen, keep the hydrogen pressure at 1.0~1.2MPa, and put the reaction The kettle was moved into an oil bath at 30°C to stir the reaction. After reacting for 6 hours, the hydrogen pressure stopped dropping, so the heating and reaction were stopped. The reaction solution was concentrated. Add 10 mL of water and 10 mL of ethyl acetate to the system, and separate the layers. The aqueous phase was extracted twice with ethyl acetate (10 mL×2), the organic phases were combined, washed once with saturated brine, and dried over anh...
Embodiment 2
[0039] Embodiment 2: Preparation of (1S)-1-(2,6-dichloro-3-fluorophenyl)ethanol
[0040] Under nitrogen protection, weigh 1.0mg (0.001mmol) chiral catalyst I (X is hydrogen) and 34mg (0.5mmol) sodium ethoxide to the reaction inner tube, add 3mL ethanol and 1035mg (5mmol) 1 -(2,6-dichloro-3-fluorophenyl)ethanone, put the reaction inner tube into the high-pressure reactor, replace the gas in the reactor with hydrogen, keep the hydrogen pressure at 1.0-1.2MPa, and move the reactor into The reaction was stirred in an oil bath at 30°C. After reacting for 6 hours, the hydrogen pressure stopped dropping, so the heating and reaction were stopped. The reaction solution was concentrated. Add 10 mL of water and 10 mL of ethyl acetate to the system, and separate the layers. The aqueous phase was extracted twice with ethyl acetate (10 mL×2), the organic phases were combined, washed once with saturated brine, and dried over anhydrous sodium sulfate. Suction filtration and spin-drying of...
Embodiment 3
[0041] Embodiment 3: Preparation of (1S)-1-(2,6-dichloro-3-fluorophenyl)ethanol
[0042] Under nitrogen protection, weigh 1.0mg (0.001mmol) of chiral catalyst I (X is 2-methoxy) and 483mg (3.5mmol) of potassium carbonate into the inner tube of the reaction, add 10ml of DMF and 1035mg of (5mmol) 1-(2,6-dichloro-3-fluorophenyl)ethanone, put the reaction inner tube into the autoclave, replace the gas in the autoclave with hydrogen, keep the hydrogen pressure at 1.0~1.2MPa, The reaction kettle was moved into a 30°C oil bath to stir the reaction. After reacting for 6 hours, the hydrogen pressure stopped dropping, so the heating and reaction were stopped. The reaction solution was concentrated. Add 10 mL of water and 10 mL of ethyl acetate to the system, and separate the layers. The aqueous phase was extracted twice with ethyl acetate (10 mL×2), the organic phases were combined, washed once with saturated brine, and dried over anhydrous sodium sulfate. Suction filtration and spi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com