Preparation method for 3-(cyclopropylmethoxy)-4-(difluoromethoxy)benzoyl chloride compound
A technology of difluoromethoxybenzoyl chloride compound and difluoromethoxybenzoic acid, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of low yield, product purity lower than 90%, and poor purity, and achieve compatibility Good, avoid sublimation loss, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
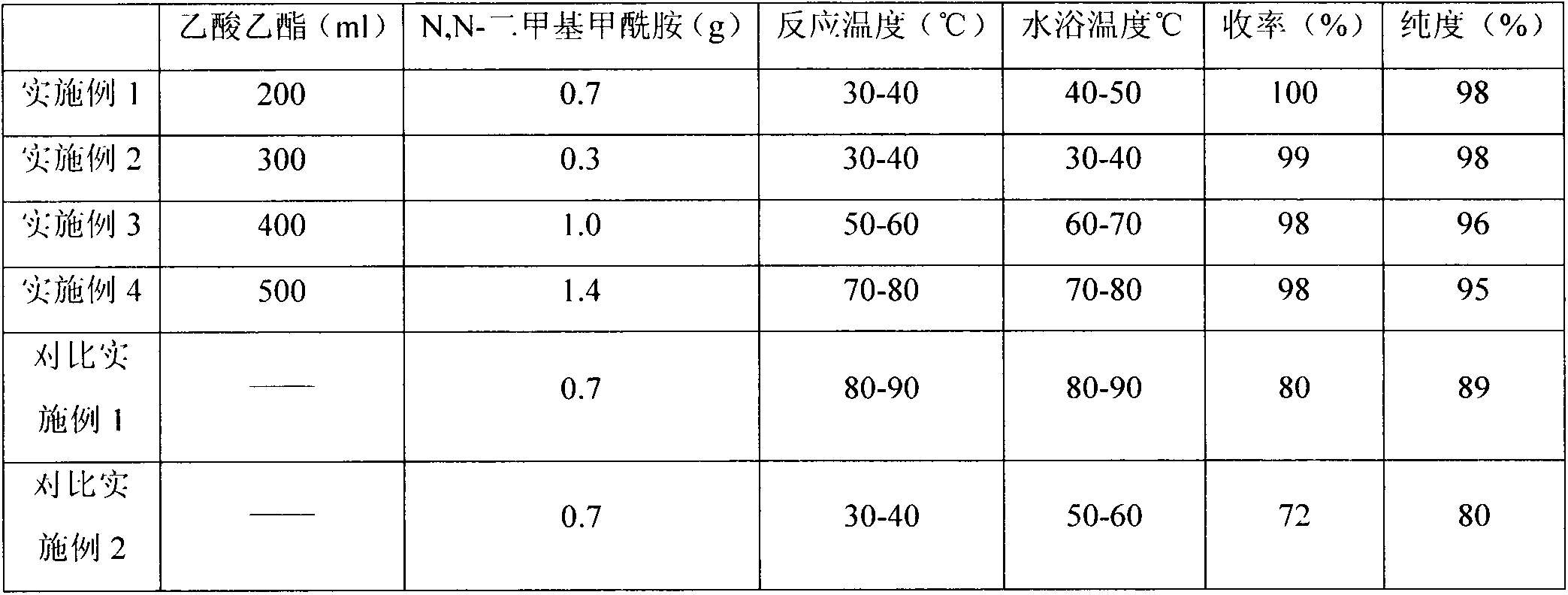
[0022] Add 50.0 g of compound 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid, 0.7 g of N, N-dimethylformamide, 200 ml of ethyl acetate into a 500 ml reaction flask, heat and stir to 30 Slowly add 34.5g of thionyl chloride dropwise at -40°C. During the dropping process, the temperature is controlled at 30-40°C. After 1 hour, the dropwise addition is completed. After the reaction was complete, excess thionyl chloride and ethyl acetate were distilled off under reduced pressure, and the temperature of the water bath was controlled at 40-50° C. to obtain 53.6 g of a solid product with a yield of 100% and a purity of 98%.
Embodiment 2
[0024] Add 50.0 g of compound 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid, 0.3 g of N, N-dimethylformamide, 300 ml of ethyl acetate into a 500 ml reaction flask, heat and stir to 30 Slowly add 34.5g of thionyl chloride dropwise at -40°C. During the dropping process, the temperature is controlled at 30-40°C. After 1 hour, the dropwise addition is completed. After the reaction was complete, the excess thionyl chloride and ethyl acetate were distilled off under reduced pressure, and the temperature of the water bath was controlled at 30-40° C. to obtain 53.0 g of a solid product with a yield of 99% and a purity of 97%.
Embodiment 3
[0026] Add 50.0 g of the compound 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid, 1.0 g of N, N-dimethylformamide, and 400 ml of ethyl acetate into a 1L reaction flask, heat and stir to raise the temperature to 50 Slowly add 34.5g of thionyl chloride dropwise at -60°C. During the dropping process, the temperature is controlled at 50-60°C. After 1 hour, the dropwise addition is completed. After the reaction was complete, the excess thionyl chloride and ethyl acetate were distilled off under reduced pressure, and the temperature of the water bath was controlled at 60-70° C. to obtain 52.5 g of a solid product with a yield of 98% and a purity of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com