Two crystal forms of azilsartan and preparation method thereof
A crystal form and X-ray technology, applied in the field of medicinal chemistry, can solve the problems of significant antihypertensive effect, and achieve the effects of long-term preservation, good fluidity, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of Azilsartan Form B:
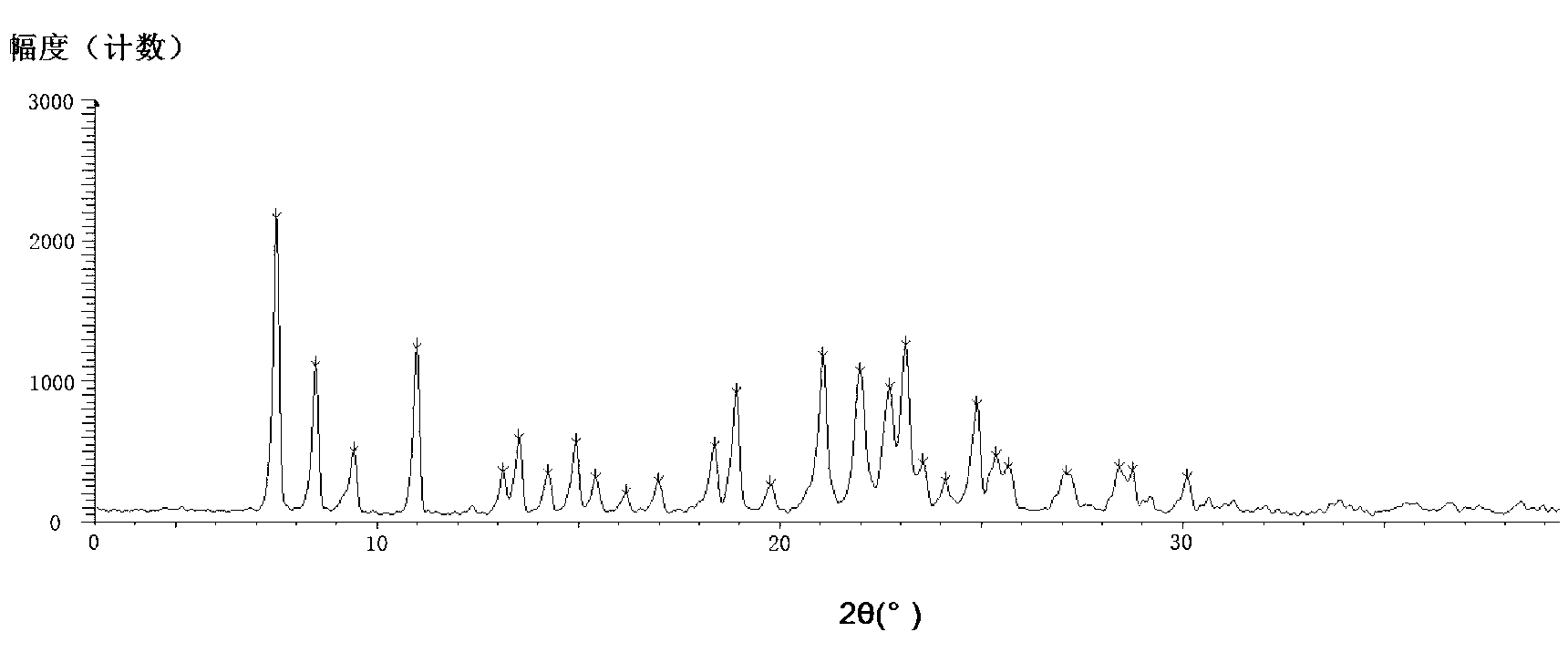
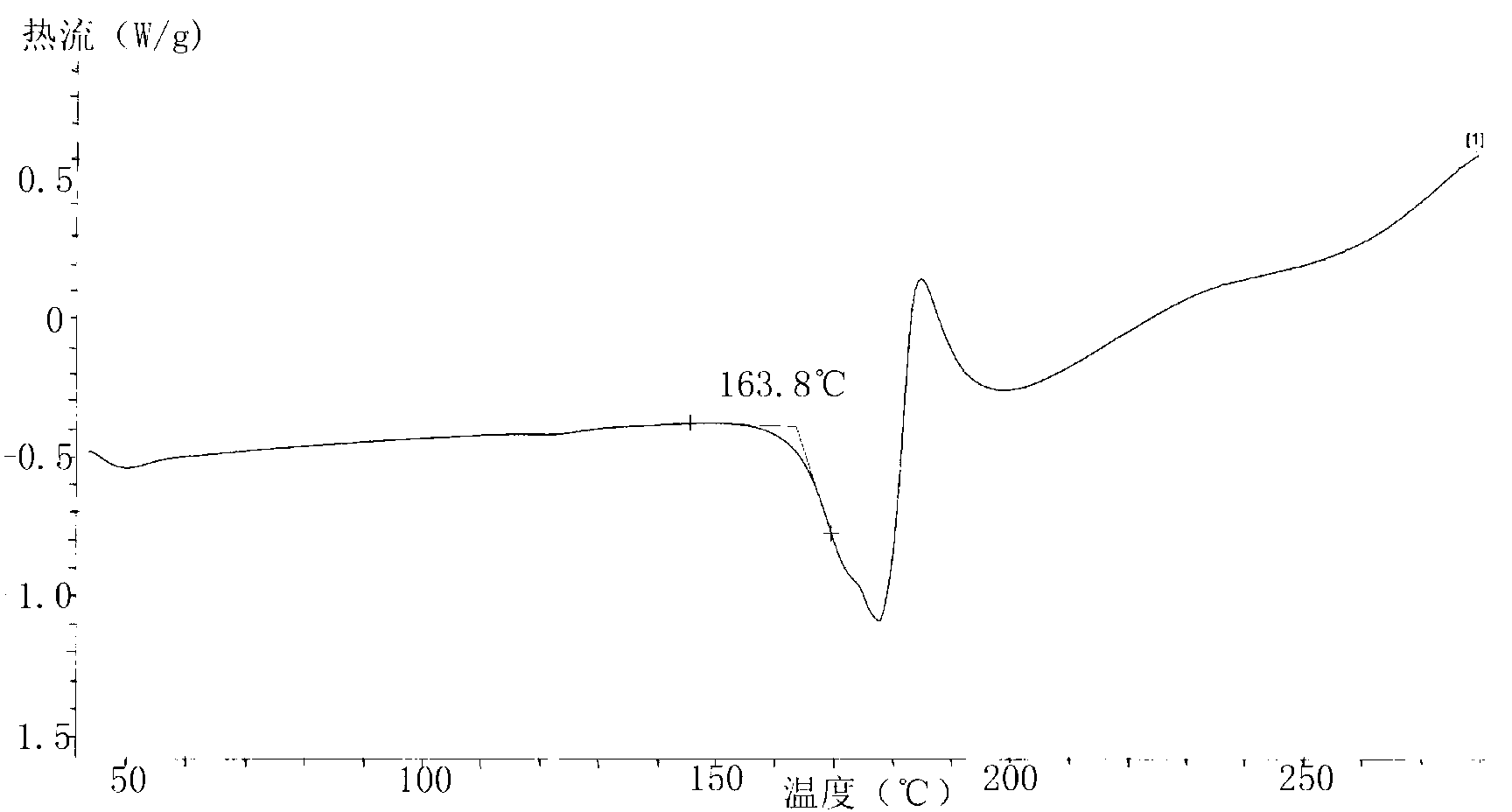
[0021] Add 3g of azilsartan into a 50ml eggplant-shaped bottle, add 3ml of DMF and 18ml of acetone, stir electromagnetically, heat to 65°C to completely dissolve, stop heating and stirring, cool to room temperature for about 30 minutes, and place at room temperature for 2 hours, then Place at 0-5°C for 5 hours. After suction filtration, the filter cake was washed with a small amount of acetone, and vacuum-dried at 35° C. for 12 hours to obtain 2.3 g of white crystals.
[0022] X-ray powder diffraction pattern see figure 1 , see the DSC analysis chart figure 2 .
Embodiment 2
[0024] Preparation of Azilsartan Form C:
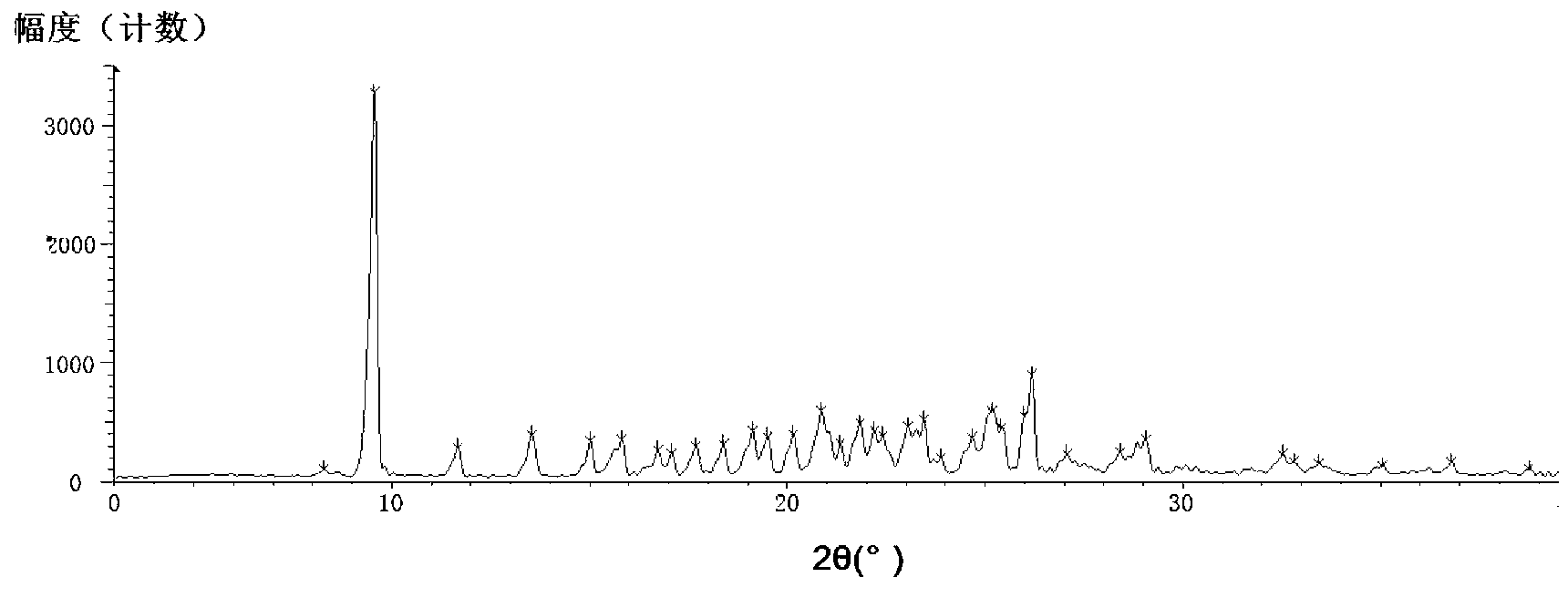
[0025] Add 3g of azilsartan into a 25ml eggplant-shaped bottle, add 3ml of DMF, stir electromagnetically and heat to 85°C to dissolve, add isopropanol dropwise to saturation (12ml), stop heating and stirring, and cool to room temperature for 30 minutes. During the cooling process, a small amount of solid precipitated out, and after standing at room temperature for 2 hours, it was left overnight at 0-5°C. After suction filtration, the filter cake was washed with a small amount of isopropanol, and vacuum-dried at 35° C. for 12 hours to obtain 2.5 g of white crystals.
[0026] X-ray powder diffraction pattern see image 3 , see the DSC analysis chart Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com