HBV phenotype drug resistance detection kit and preparation method thereof
A detection kit and drug resistance technology are applied in the preparation of HBV phenotype drug resistance detection kits and the field of phenotype drug resistance detection kits, achieving the effects of high sensitivity and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1HB
[0044] The construction of embodiment 1HBV phenotype drug-resistant carrier
[0045] The construction steps of the HBV phenotypic drug resistance vector include producing a set of Gateway-based TM Recombinant expression vector system. The system includes a dual expression vector with secreted alkaline phosphatase and a recombinant entry vector that can replace HBV1.0 fragments. The main technical routes are as follows: Figure 8 shown.
[0046] Specific steps are as follows:
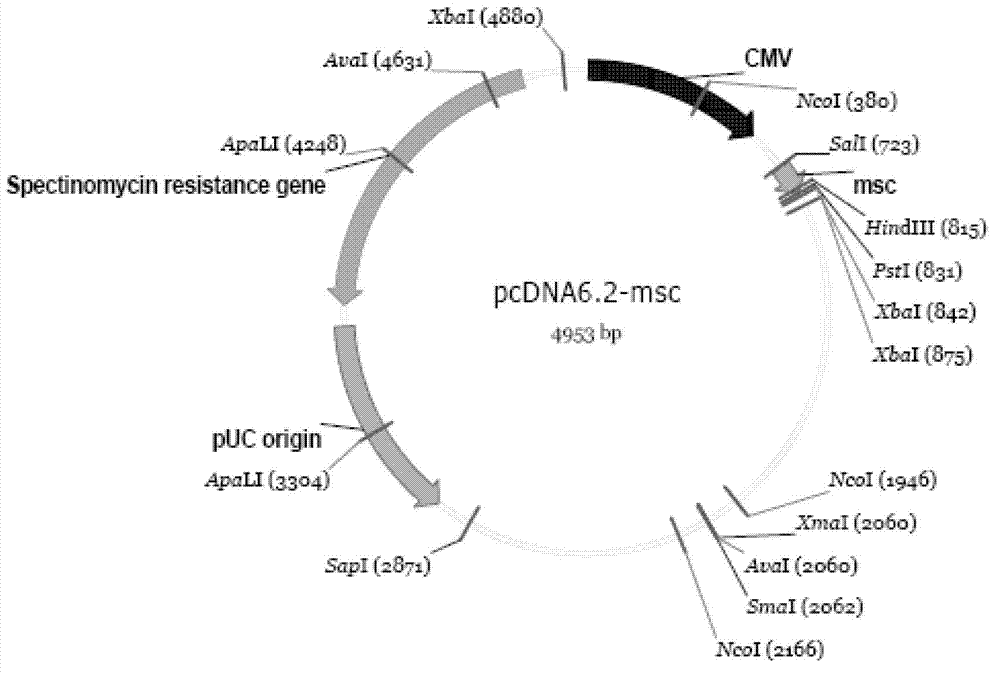
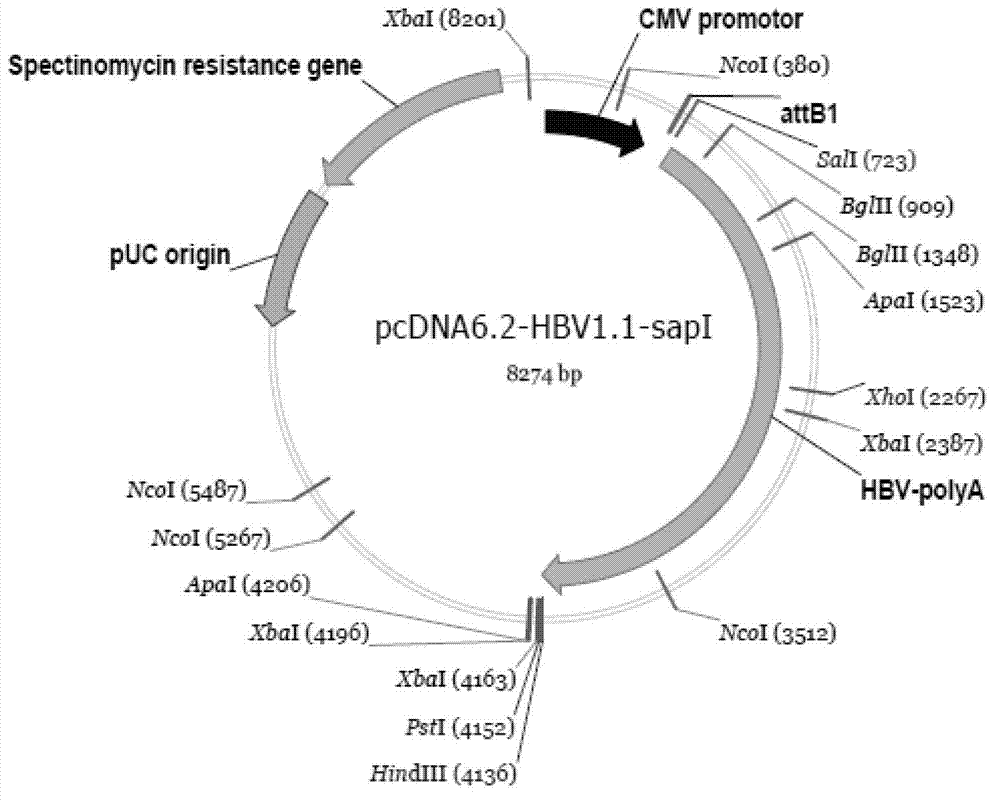
[0047] 1. Construction of pcDNA6.2-HBV1.1-sapI vector that can replace HBV1.0 fragment
[0048] The steps are specifically:
[0049] 1.1 First, amplify the multiple cloning site region of the pMD18T self-ligating vector, and the primers are as follows;
[0050] 6.2-salI-s: 5'-ACGCGTCGACCGAAAGGGGGATGTG-3' (shown in SEQ ID No.8);
[0051] 6.2-salI-as: 5'-ACGCTCGAGGAGCTCGGTACCCGG-3' (shown in SEQ ID No.9);
[0052] Use the pMD18T self-ligating carrier stored in our laboratory as a template for amplif...
Embodiment 2HB
[0071] Example 2 HBV drug resistance carrier system drug resistance detection application verification
[0072] 1. Substitution of the HBV1.0 fragment to be tested was introduced into the pcDNA6.2-HBV1.1-sapI vector constructed in Application Example 1 to form the entry clone pcDNA6.2-HBV1.1(p);
[0073] 1.1 Amplification of standard HBV C1 subtype HBV1.0 fragment
[0074] Primers used: HBV1.0-as: TAGTGGAAGCTTGACCATGGTGAGCAAGGGCGAG (shown in SEQ ID No.16);
[0075] HBV1.0-s: GCTTGGGATCCAATTCTTACTTGTACAGCTCG (shown in SEQ ID No.17);
[0076] Preserve the HBV C2 subtype plasmid template in the laboratory, reaction conditions: 94 degrees for 2 minutes; 94 degrees for 15 seconds, 55 degrees for 30 seconds, 72 degrees for 3 minutes and 20 seconds for 30 cycles of amplification; 72 degrees for 10 minutes;
[0077] 1.2 The amplified product and the pcDNA6.2-HBV1.1-sapI vector were digested with sapI and SalI and ligated to form pcDNA6.2-HBV1.1(p).
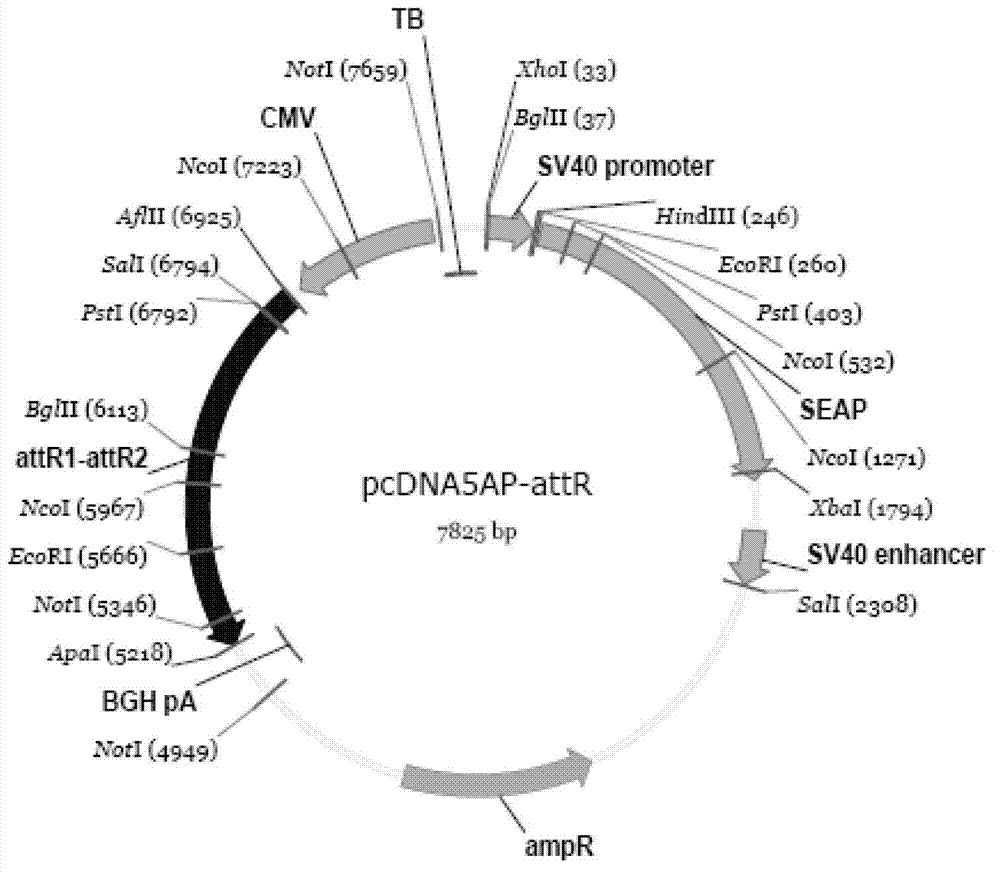
[0078] 2. Recombination: clonin...
Embodiment 3
[0085] Embodiment 3 Description about the kit
[0086] In the kit provided by the present invention, other components or structures such as primers, probes, chromogenic reagents and the like are also included. The specific instructions are as follows:
[0087] pcDNA6.2-HBV1.1-sapI vector 1 μg
[0088] pcDNA5AP-attR vector 1 μg
[0089] Primer HBV1.0-as TAGTGGAAGCTTGACCATGGTGAGCAAGGGCGAG (shown in SEQ ID No.16)
[0090] Primer HBV1.0-s GCTTGGGATCCAATTCTTACTTGTACAGCTCG (shown in SEQ ID No.17)
[0091] Other components of the kit, such as reagents for amplification PCR, etc., can be synthesized and purchased by themselves, and will not be described in detail here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com