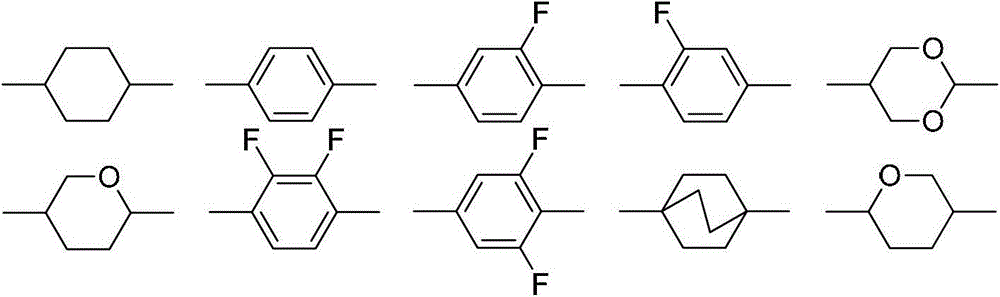
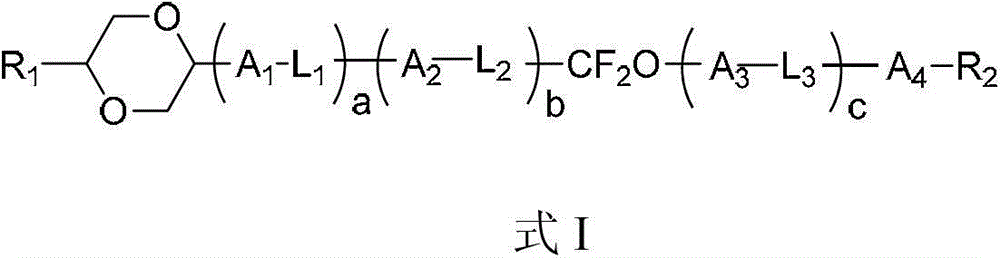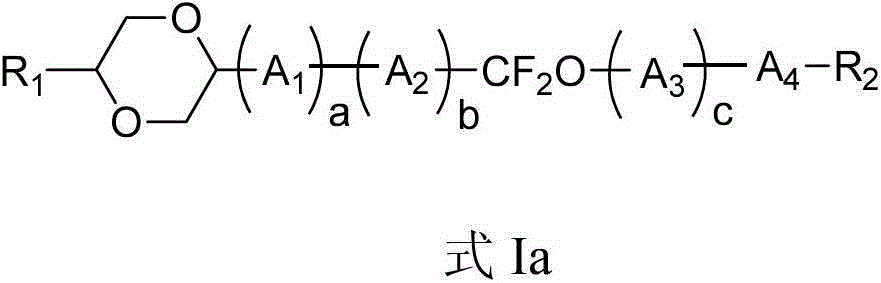Liquid-crystal compound containing 1,4-dioxo-six-membered ring as well as preparation method and application thereof
A compound and liquid crystal technology, applied in the field of containing 1, can solve the problems of greatly reducing the liquid crystal clearing point and restricting the space for increasing the response speed of the liquid crystal mixture.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1, compound shown in preparation formula I
[0087]
[0088] step 1:
[0089]
[0090] Add 56.64g (0.24mol) of 1,4-bromobenzene (reactant) and 400ml of dry tetrahydrofuran (solvent) into a 1L three-necked flask. ) n-butyllithium (reactant), dropwise and keep warm for 1 hour, under the same temperature, drop 42.6g (0.216mol, 72%) 1,4-dioxane-2-ketone (reactant) and 50ml dry The mixed solution of tetrahydrofuran (solvent) was stirred for 30 minutes after dropping, and the temperature was raised naturally, and 200ml of saturated ammonium chloride aqueous solution was added dropwise at about 0°C (adjusting the pH value), and the liquid was separated, and the aqueous phase was extracted with 200ml of ethyl acetate (solvent). The phase was washed with water, spin-dried to obtain 54g (GC: 89%) liquid, and another 1L three-necked flask was added with 54g of the product obtained above, 500ml of dry dichloromethane (solvent), and cooled to -25~-20°C under nitro...
Embodiment 2
[0109]
[0110] step 1:
[0111]
[0112] Add 60.96g (0.24mol) of 1,4-dibromo-2-fluorobenzene (reactant) and 400ml of dry tetrahydrofuran (solvent) into a 1L three-necked flask. 0.24mol, 2.5N) n-butyllithium (reactant), dropwise insulation 1 hour, under the same temperature, drop into 42.6g (0.216mol, 72%) 1,4-dioxane-2-ketone (reaction substance) and 50ml dry tetrahydrofuran (solvent) mixed solution, after dropping, stir for 30 minutes, naturally warm up, add 200ml saturated ammonium chloride aqueous solution (adjust pH value) dropwise at about 0°C, separate liquids, use 200ml ethyl acetate ( solvent) extraction, the organic phase was washed with water, spin-dried to obtain 54g (GC: 89%) liquid, in another 1L there-necked flask, add 54g of the product obtained above, 500ml dry dichloromethane (solvent), under nitrogen protection, cool to- 25~-20 ℃, dropwise add 63.3ml (0.397mol, 2.2eq) triethylsilane hydrogen (reactant), dropwise, add dropwise 50ml (0.397mol, 2.2eq) b...
Embodiment 3
[0134] Embodiment 3, preparation formula I described compound
[0135]
[0136] step 1
[0137]
[0138] Same as above embodiment 1, step 1.
[0139] step 2
[0140]
[0141] Add 0.1mol (3-a) (reactant) and 120ml tetrahydrofuran (solvent) obtained in the step into the reaction flask, install and seal the stirring, replace the air with nitrogen, cool to -70°C, and add dropwise 0.1mol concentration of 2.5M butane Lithium-based (reactant), 20 minutes after the addition, feed dry carbon dioxide gas (reactant), to saturation, react at this temperature for 2 hours, pour this reaction solution into 20ml concentrated hydrochloric acid (adjust pH value) Hydrolyze in a beaker with 100ml of water, separate the liquids, extract the water phase once with 50ml of ethyl acetate (solvent), combine the organic phases, wash with saturated brine until neutral, dry over anhydrous sodium sulfate (desiccant), concentrate to remove the solvent, and obtain Pale yellow solid, recrystalliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com