Metal organic framework modified materials, methods of making and methods of using same
A modification, organic fiber technology, applied in the field of metal-organic framework modified materials, and its preparation and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
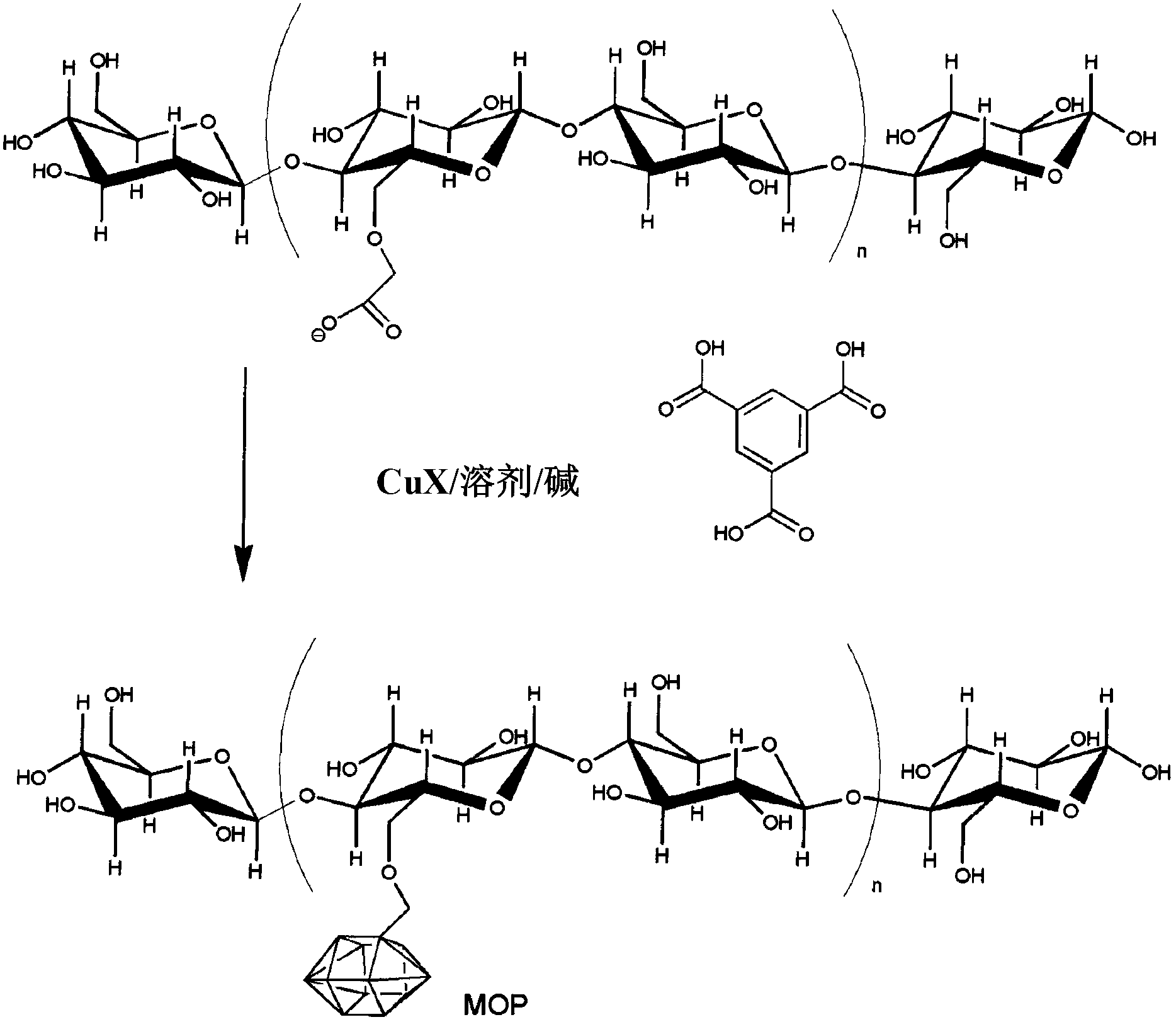
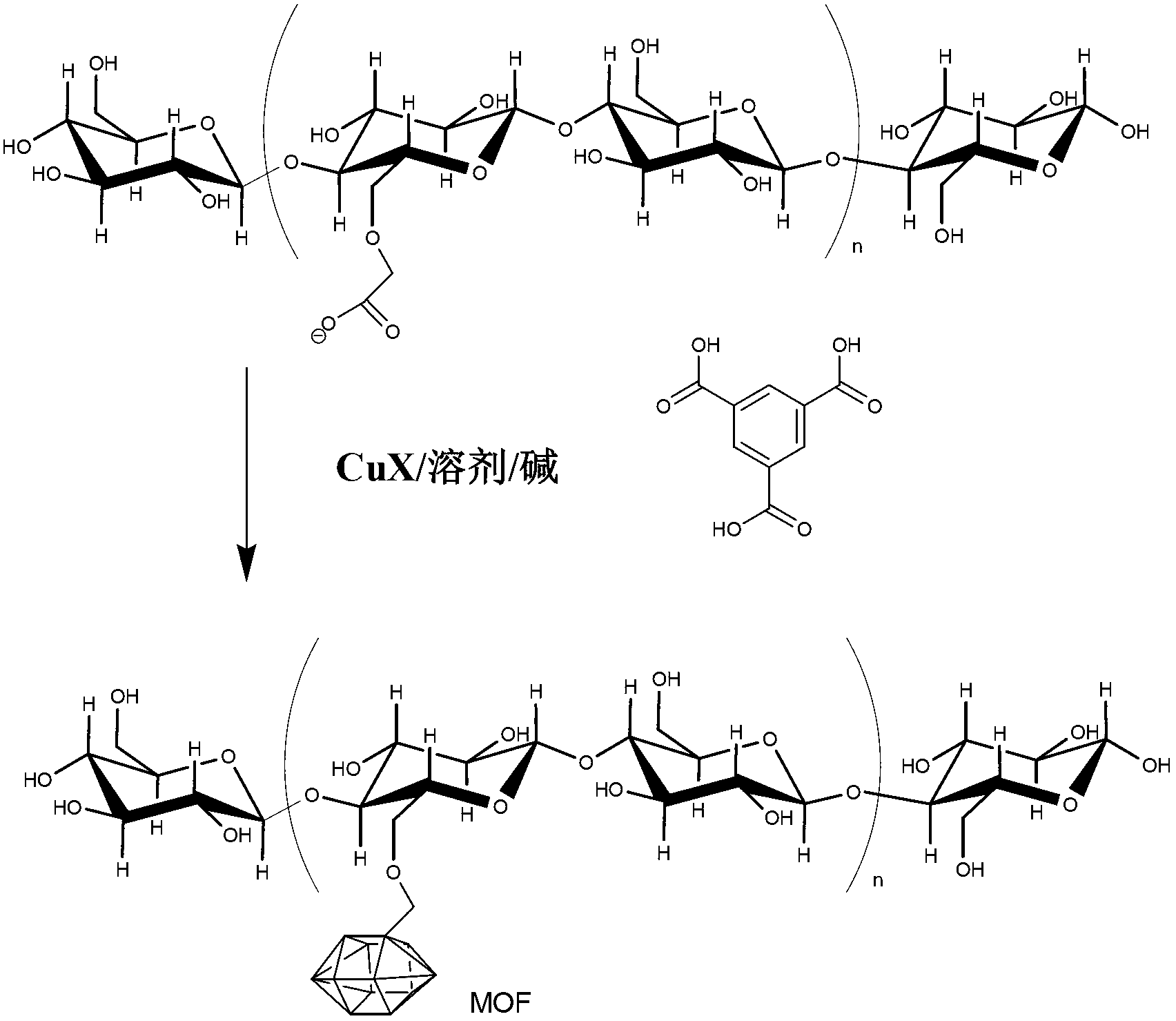
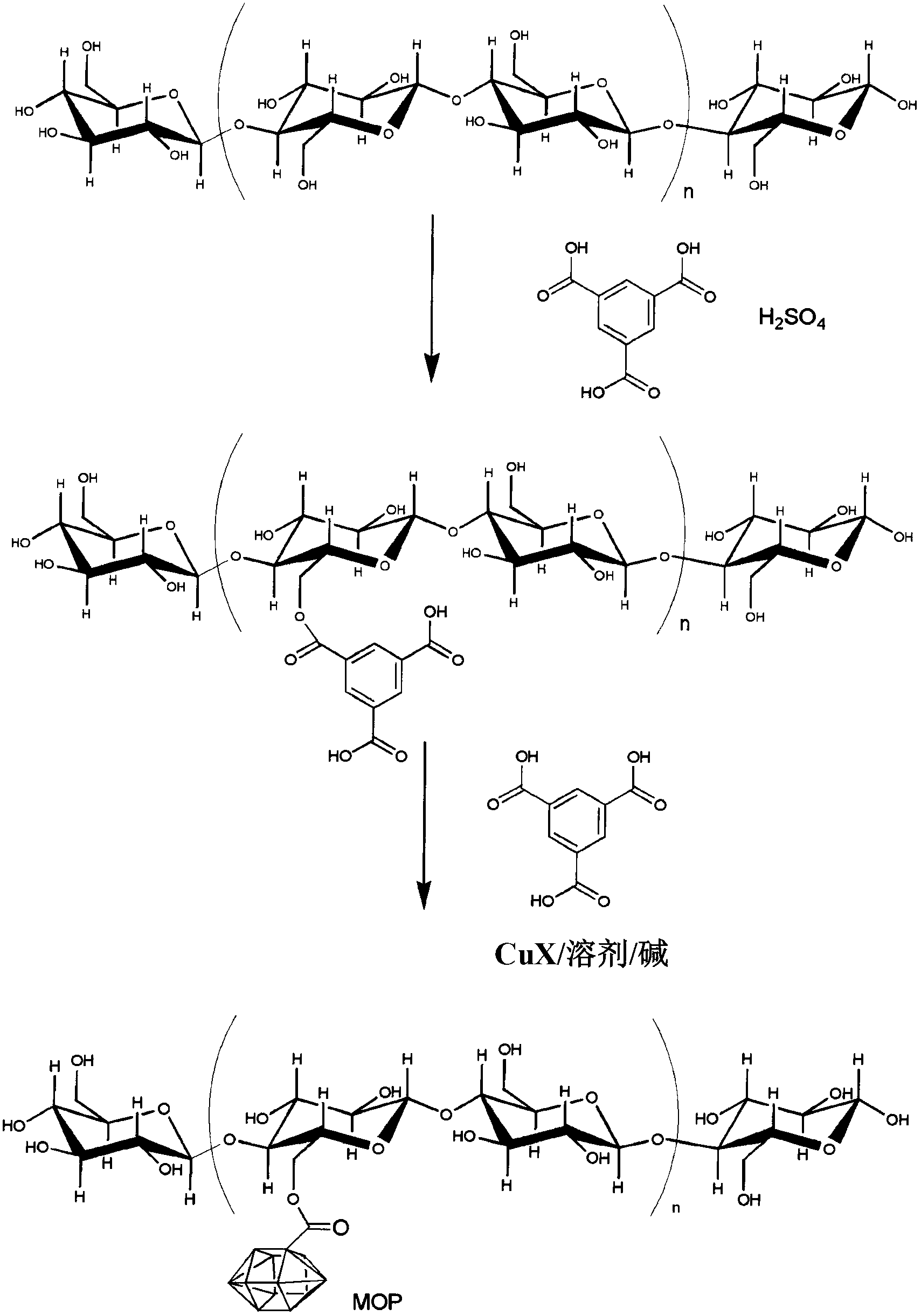
[0068] In one embodiment, the method for preparing MOF-modified fibers includes the steps of: providing a suitable material; and exposing the material to MOF under conditions that allow the formation of the MOF-modified material. For example, Figure 1 shows the method of MOF199 incorporation into anionically modified cellulose fibers.
[0069] In another embodiment, a method for preparing a MOF-modified material includes the steps of: providing a suitable material; and exposing the material to a mixture of MOF precursors under conditions that allow the formation of a MOF-modified material. In this embodiment, the MOF is synthesized in situ in the presence of the material such that the MOF is covalently bound to the fiber.
[0070] In the method of the present invention, appropriate reaction conditions should be determined (for example, reagent amount, solvent, reaction temperature, and reaction pressure are all within the consideration of those skilled in the art).
[0071] I...
Embodiment 1
[0089] General experiment: Prepare chemically modified cellulose (anionic cellulose) before the experiment. Copper(II) acetate, 1,3,5-benzenetricarboxylic acid, methanol (MeOH), dimethylformamide (DMF), and ethanol (EtOH) were obtained from Sigma-Aldrich (St. Louis, MO). All chemicals and reagents used were of analytical grade. Scanning electron microscope (SEM) micrographs were recorded using a scanning electron microscope (SEM, LEO1550FE-SEM) at 20 kV, and the surface was coated with carbon before analysis. Powder X-ray diffraction patterns were collected using an X-ray diffractometer in Rigaku SmartLab. Thermogravimetric analysis (TGA) using a thermogravimetric analyzer at N 2 And the analysis is carried out under the conditions of 30°C to 600°C. Fourier transform infrared spectroscopy (FT-IR) analysis was performed using a Nicolet Magna 760 FTIR spectrometer (Thermo Fisher Scientific Inc., Waltham, MA) in single attenuated total reflectance (ATR) mode. X-ray photoelect...
Embodiment 2
[0095] experiment:
[0096] A-Cu(OAc) 2 (DMF / EtOH / H 2 O), anionic cellulose
[0097] B-anionic cellulose, Cu(OAc) 2 (DMF / EtOH / H 2 O), 1,3,5-Benzenetricarboxylic acid (DMF / EtOH / water)
[0098] C-Cu(OAc) 2 (DMF / EtOH / H 2 O), 1,3,5-benzenetricarboxylic acid (DMF / EtOH / H 2 O), anionic cellulose
[0099] D-anionic cellulose, Cu(OAc) 2 (DMF / EtOH / H 2 O) 1,3,5-benzenetricarboxylic acid (DMF / EtOH / H 2 o) overnight / stirring
[0100] A-1,3,5-Benzenetricarboxylic acid (DMF / EtOH / H 2 O) and triethylamine
[0101] B-triethylamine
[0102] C-1,3,5-Benzenetricarboxylic acid (DMF / EtOH / H 2 O)
[0103] D- no alkali
[0104] washing:
[0105] distilled water
[0106] DMF
[0107] MeOH
[0108] stir for 5 hours
[0109] Chemical attachment of MOF199 to anionically modified anionic cellulose. MOF199 was synthesized in situ using a modified known method. A different experimental procedure was performed to bind MOF199 to the modified cellulose. A and C: Copper(II) acetate (860mg)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com