Method for separating and purifying beta-end-group thio-glycoside compound
An end-group thioglycoside and purification method technology, which is applied in the field of separation and purification of beta-end-group thioglycoside compounds, can solve the problems of long separation time, complicated operation and the like, and achieve the effect that the separation and purification method is simple and quick.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of thioglycoside compounds with reference to the Chinese patent application number 201210114627:
[0037] a) Add 640ml of 33wt% acetic acid / HBr solution to 607g of acetylated fucose, stir to dissolve, react for about 1.5h, and test the reaction progress by TLC spot plate; the reaction is considered complete when the raw material point disappears; then add to the reaction solution Add 1.9L of dichloromethane, then add 5wt% Na 2 CO 3 863ml of the solution was used to neutralize the pH value to 7, and the organic phase was separated by stirring. The organic phase was dried with anhydrous sodium sulfate and then evaporated to dryness at 40°C to obtain bromoacetylated fucose with a theoretical yield of 645g.
[0038]b) Add 480g of bromoacetylated fucose to 2.5L of acetonitrile to dissolve it, and heat it to 70°C; slowly add 144.5g of thiourea to the reaction solution, and white flocs will form when the reflux begins; After adding thiourea, react for about 30 mi...
Embodiment 2
[0041] Dissolve 50 g of the crude thiofucoside compound obtained in Example 1 into 100 ml of dichloromethane, add 100 ml of an aqueous solution of copper sulfate with a mass fraction of 5%, stir at -20°C for 30 min, separate the organic phase by filtration, and heat to 40 degrees, dichloromethane was evaporated to obtain an oily product. Add 100ml of a mixture of methyl tert-butyl ether and isohexane (1:2 volume ratio of methyl tert-butyl ether and isohexane) to the oily product, and put the device containing the above product into ultrasonic vibration until all crystals are precipitated . Filtration and washing with isohexane gave 18.5 g of crystals with a purity of 98.5%, with a yield of 91%.
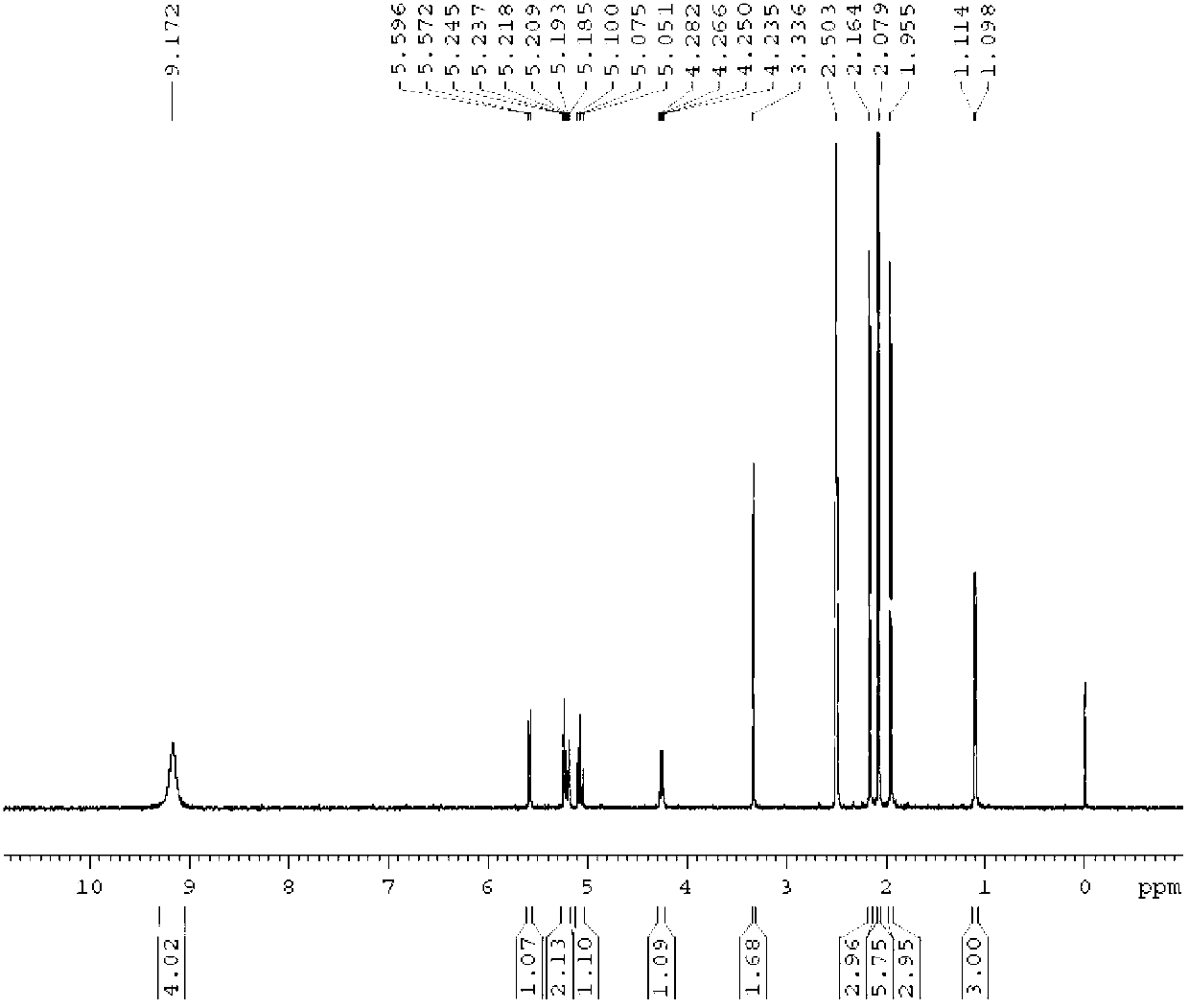
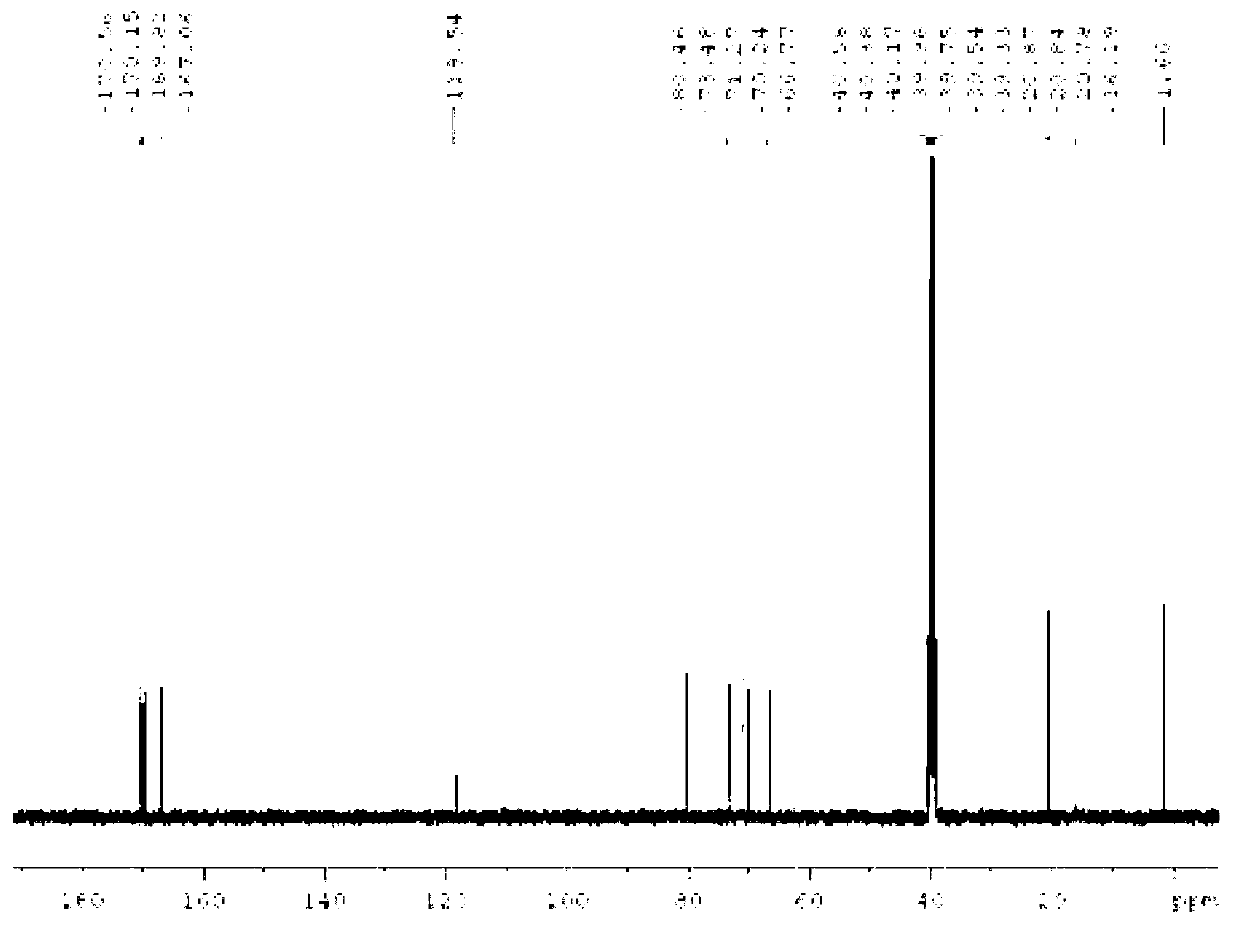
[0042] Experimental analysis was carried out on the obtained crystals, figure 1 It is the hydrogen nuclear magnetic resonance spectrum of the β-terminal thiofucoside compound, figure 2 It is the carbon nuclear magnetic resonance spectrum of the β-terminal thiofucoside compound, co...
Embodiment 3
[0044] Dissolve 100g of the crude thiofucoside compound obtained in Example 1 into 200ml of dichloromethane, add 200ml of copper sulfate solution with a mass fraction of 8%, stir at -15°C for 40min, separate the organic phase, and heat to 40 degree, dichloromethane was evaporated to obtain an oily product. Add 250ml of a mixture of methyl tert-butyl ether and isohexane (volume ratio 1:3) to the oily product, and put the device containing the above product into ultrasonic vibration until all crystals are precipitated. Filter and wash with isohexane to obtain 37.8 g of crystals with a purity of 98.2%, that is, β-terminal thioglycoside compounds, with a yield of 92.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com