High-performance polyazo green reactive dye and synthesis and application of dye mixture
A technology of reactive dyes and polyazo, which is applied in the field of synthesis of reactive polyazo dyes, can solve the problems of high-temperature dyes that are not energy-saving, high one-time success rate, and reduce printing and dyeing costs, and achieve good washability, energy saving, Bright and full effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The preparation of embodiment 1 polyazo navy blue dye Ⅰ-1
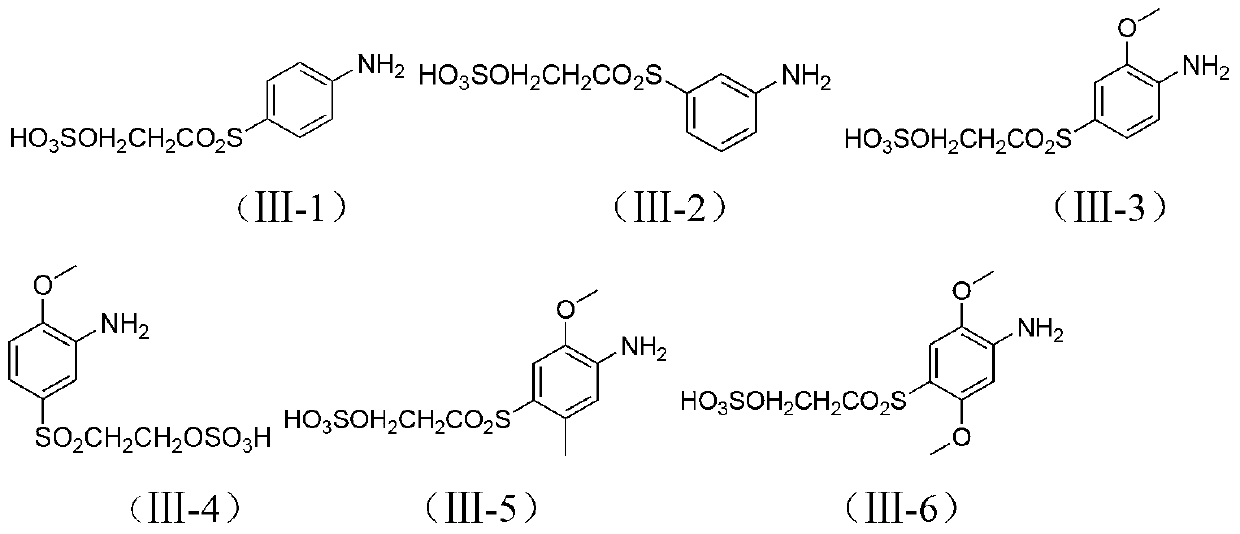
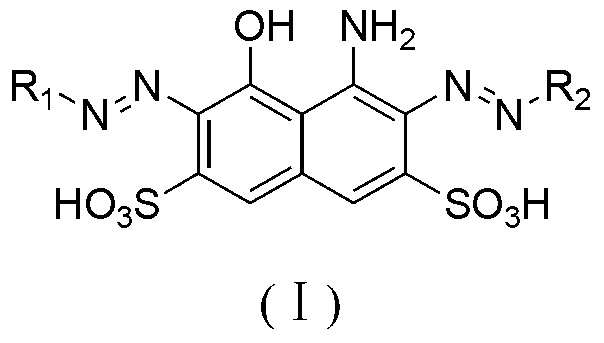
[0062] In a 1000ml beaker, add 150 parts of water, 28.1 parts of para-ester, 0.1 part of dispersant, stir and beat for 2 hours, add 30 parts of 30% hydrochloric acid, and add 23 parts of 30% sodium nitrite at 0-5 °C The solution was subjected to diazotization reaction for 2 hours. After reaching the end point, sulfamic acid was added to destroy excess nitrous acid, then 341 parts of H acid was added, the temperature was raised to 10-15°C, and the pH was adjusted to 2-2.5 with 15% sodium acetate solution. React for 4 to 6 hours, and when there is no diazonium salt, it is the end point, and it is set aside.
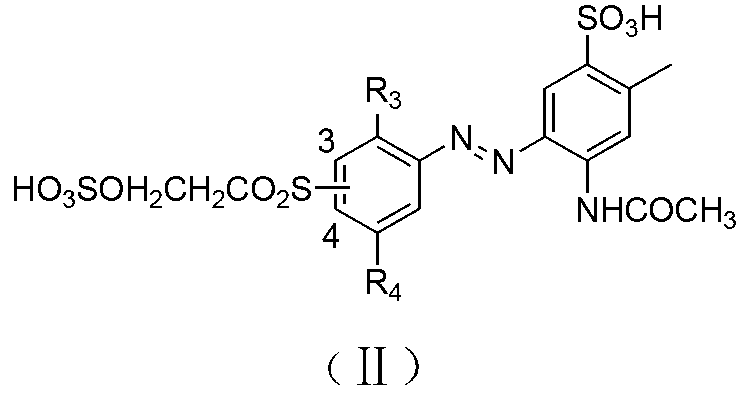
[0063] In a 1000 ml beaker, add 350 parts of water, 52.3 parts of the compound of formula Ⅱ-1 (R 3 , R 4 hydrogen), 0.1 part of dispersant, stir until fully dissolved, add 30 parts of 50% sulfuric acid, add 23 parts of 30% sodium nitrite solution at 0-5°C for diazotization reaction for 2 hours, and add Su...
Embodiment 2-5
[0065] Example 2-5 Preparation of polyazo dark blue and green dyes Ⅰ-2~Ⅰ-5
[0066] With reference to the method of Example 1, dyes I-2 to I-5 can be obtained. Compared with Example 1, the only difference is that the substituents of the compounds of formula II and formula III in the reaction process are different.
[0067]
Embodiment 6-11
[0068] The preparation of embodiment 6-11 polyazo green dye I-6~I-11
[0069] Change the coupling order of the diazo compound of formula II and formula and H acid in a similar manner to Example 1, that is, the diazo compound of formula II is first acidicly coupled with acid H, and then the diazo compound of formula III is subjected to alkali coupling. Sexual coupling to obtain polyazo green dyes Ⅰ-6~Ⅰ-11.
[0070]
[0071]
[0072] Only some dyes of the general formula I are exemplified here, and those skilled in the art can foresee that dyes I with different substituents can be obtained by using different reactants to finally realize the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com