Kit for rapid mass spectrometric detection of leptospira
A leptospira and spirochete technology, applied in the field of protein fingerprint detection, can solve the problems of no leptospira, unable to identify leptospira, unable to identify pathogenicity, etc., and achieve the effect of eliminating the hazards of personnel and the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Liquid culture Leptospira sample pretreatment optimization
[0053] Due to the complex composition of the liquid medium, there are many factors that affect the quality of the mass spectrometer, so the effective removal of the medium components of the sample before bacterial protein extraction is very critical. Bacteria washing is an effective way to effectively reduce the influence of medium components.
[0054] The formula of the bacteria washing solution A used in this example is 0.2g of potassium dihydrogen phosphate, 2.9g of disodium hydrogen phosphate, 8.0g of sodium chloride, 0.2g of potassium chloride, Tween-200.5mL, add water to 1000mL, use hydrochloric acid Adjust the pH to 7.4 and autoclave.
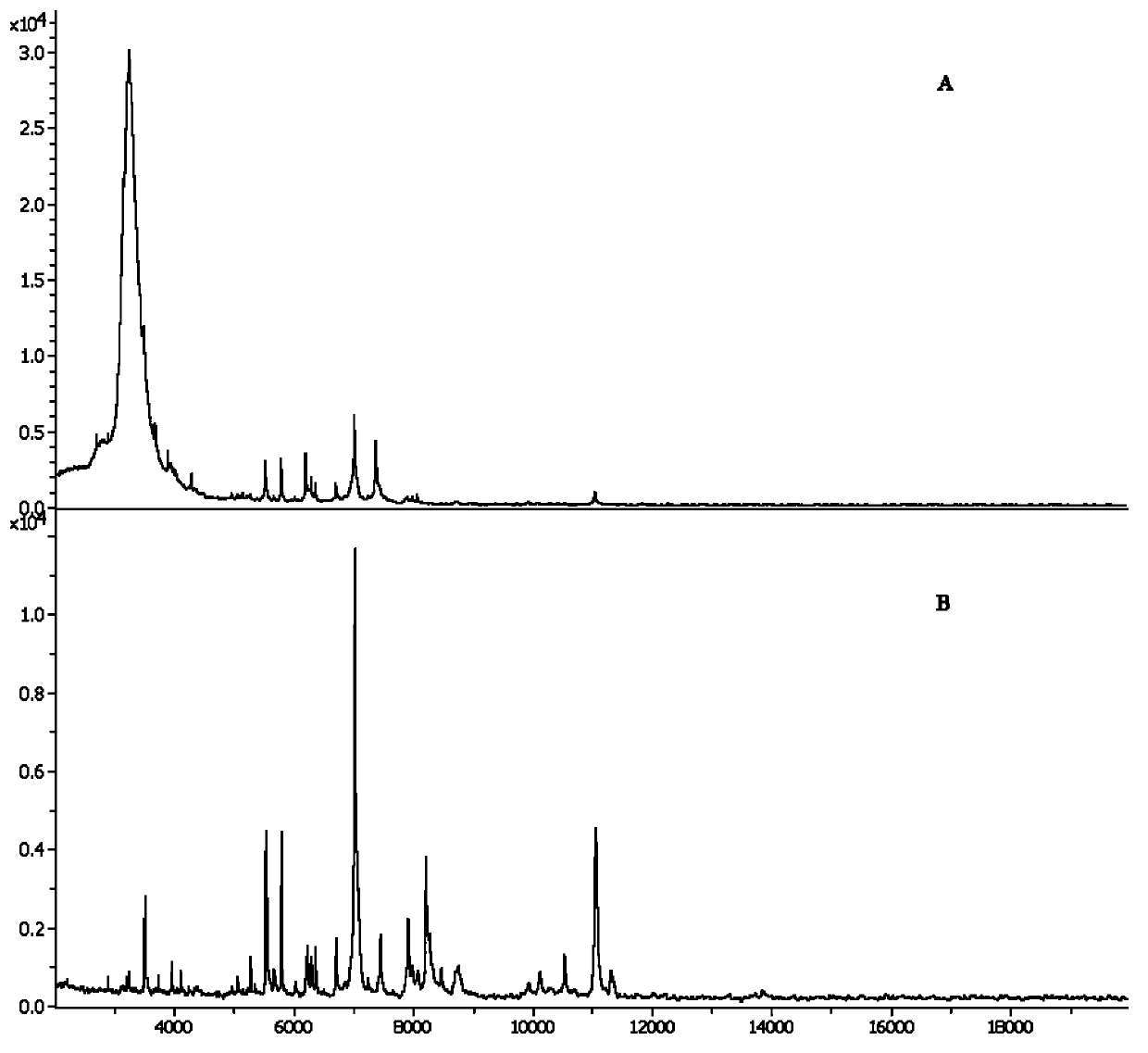
[0055] The main washing step of the bacteria is completed by the bacteria washing solution A. In this embodiment, the Leptospira are not washed with the washing solution A, and are washed once with the washing solution A for peptide fingerprint analysis. After...
Embodiment 2
[0059] Embodiment 2 Construction of Leptospira mass spectrometry model
[0060] 1. Samples and instruments
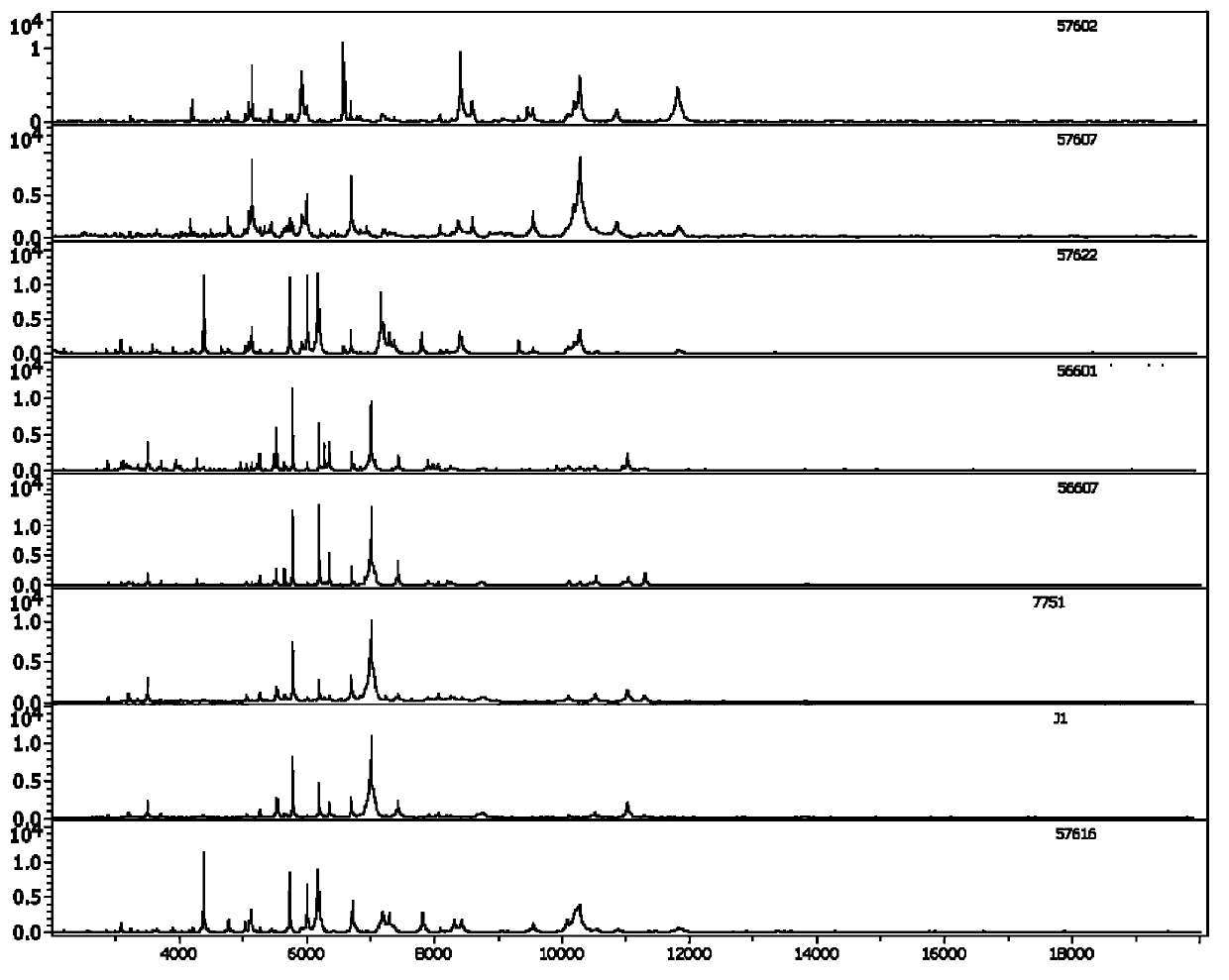
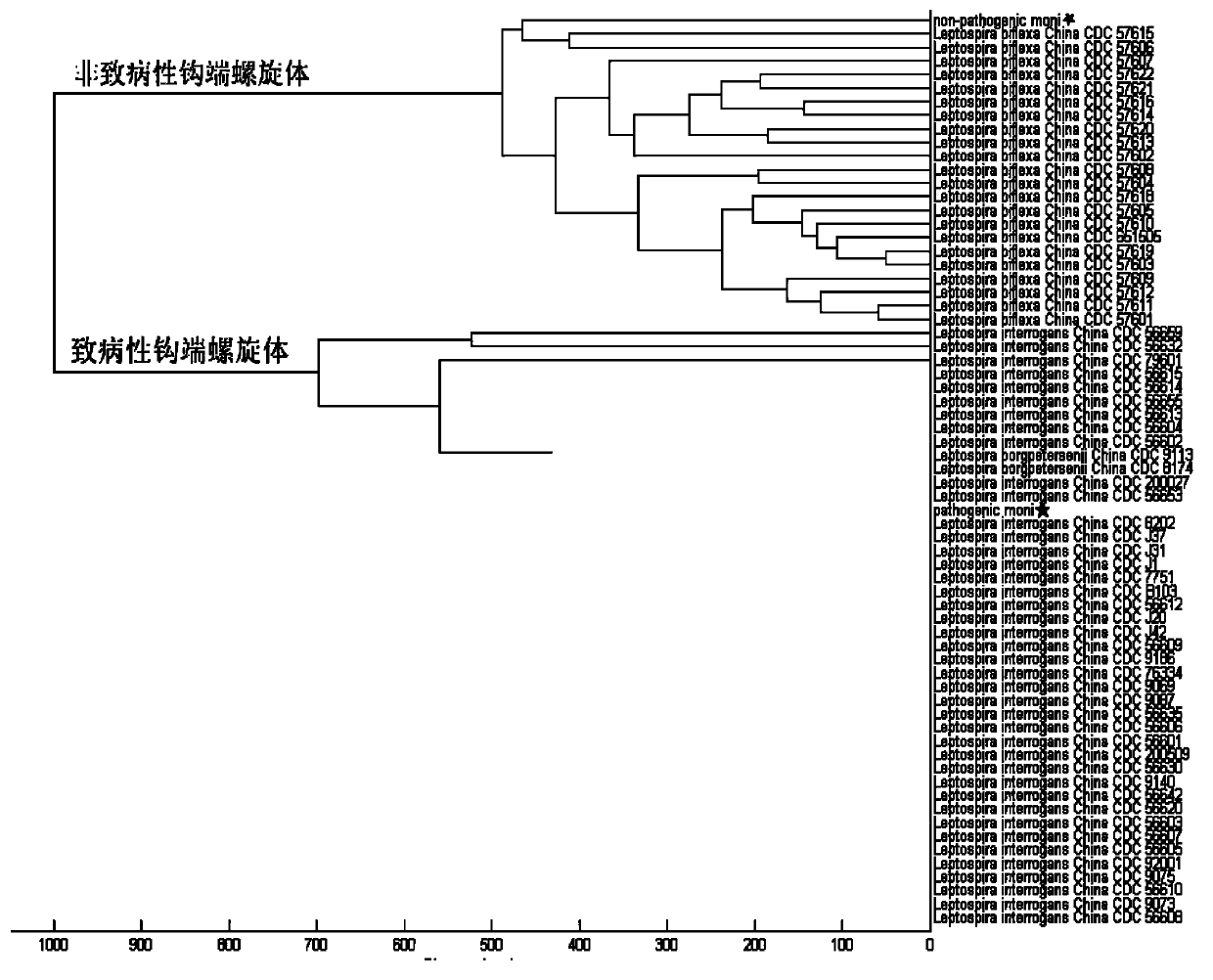
[0061] Forty-three strains of pathogenic Leptospira and 22 strains of non-pathogenic Leptospira were used, and 15 strains of ATCC international standard strains were randomly selected for the construction of mass spectrometry models (Table 1). The remaining 35 strains were used for model testing. All strains have been confirmed pathogenicity by PCR using G1 / G2, B64-I / B64-II as primers and 16SrRNA.
[0062] Table 1. List of strains for model construction
[0063]
[0064] 2. Cell pretreatment
[0065] Take 5 mL of Leptospira bacterial suspension cultured to the logarithmic growth phase, centrifuge at 12000g for 5min, discard the upper liquid medium; wash the bacterial cell sediment with cell washing solution A at 12000g, centrifuge for 5min, and discard the supernatant; Add 200 μL bacterial cell washing solution B, mix well, add 600 μL bacterial cell washing solut...
Embodiment 3
[0074] Example 3 Leptospira mass spectrometry model verification
[0075] Sensitivity (sensitivity), also known as true positive rate (true positive rate), is actually the pathogen and is correctly judged as the percentage of the pathogen according to the standard of the detection method. Specificity, also known as true negative rate, is the percentage that is actually not the pathogen but is correctly judged as the pathogen according to the standard of the detection method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com