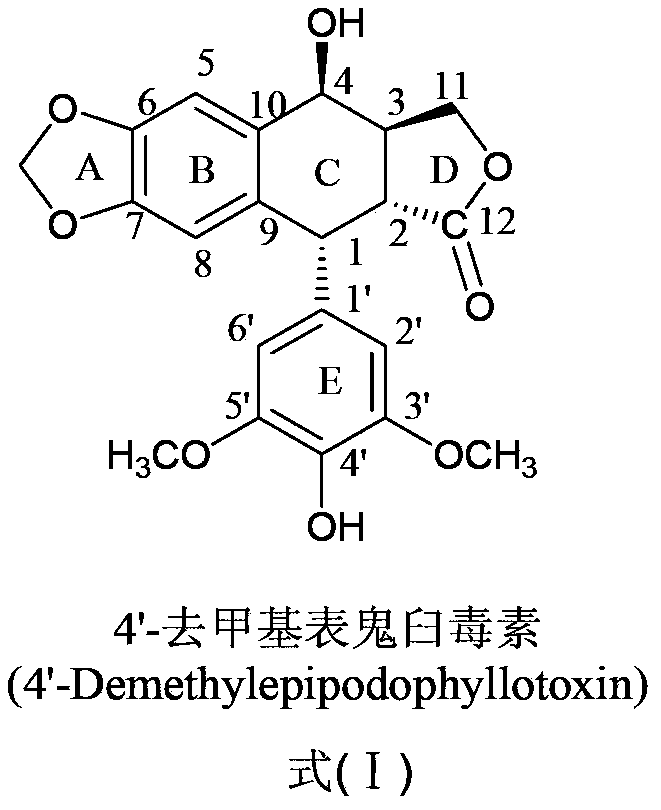
Esterified podophyllum derivative with antineoplastic activity and preparation method and application thereof
A technology of anti-tumor activity and derivatives, applied in the direction of anti-tumor drugs, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of large toxic and side effects, limited use, poor bioavailability, etc., and achieve anti-tumor Increased activity and good anti-tumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
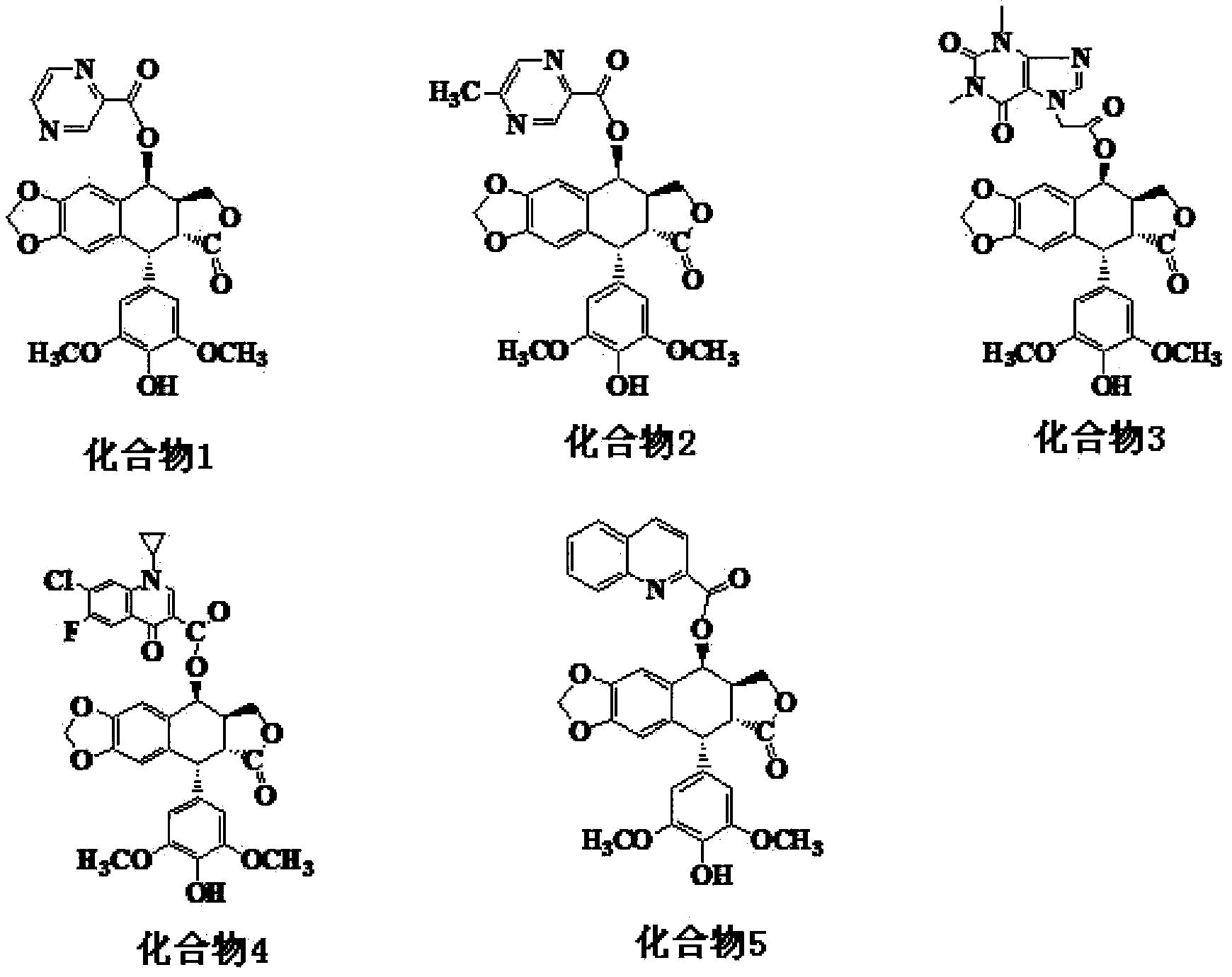
[0036] Example 1 Synthesis and purification of 4-O-(2-pyrazinecarboxylic acid-1)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound 1)
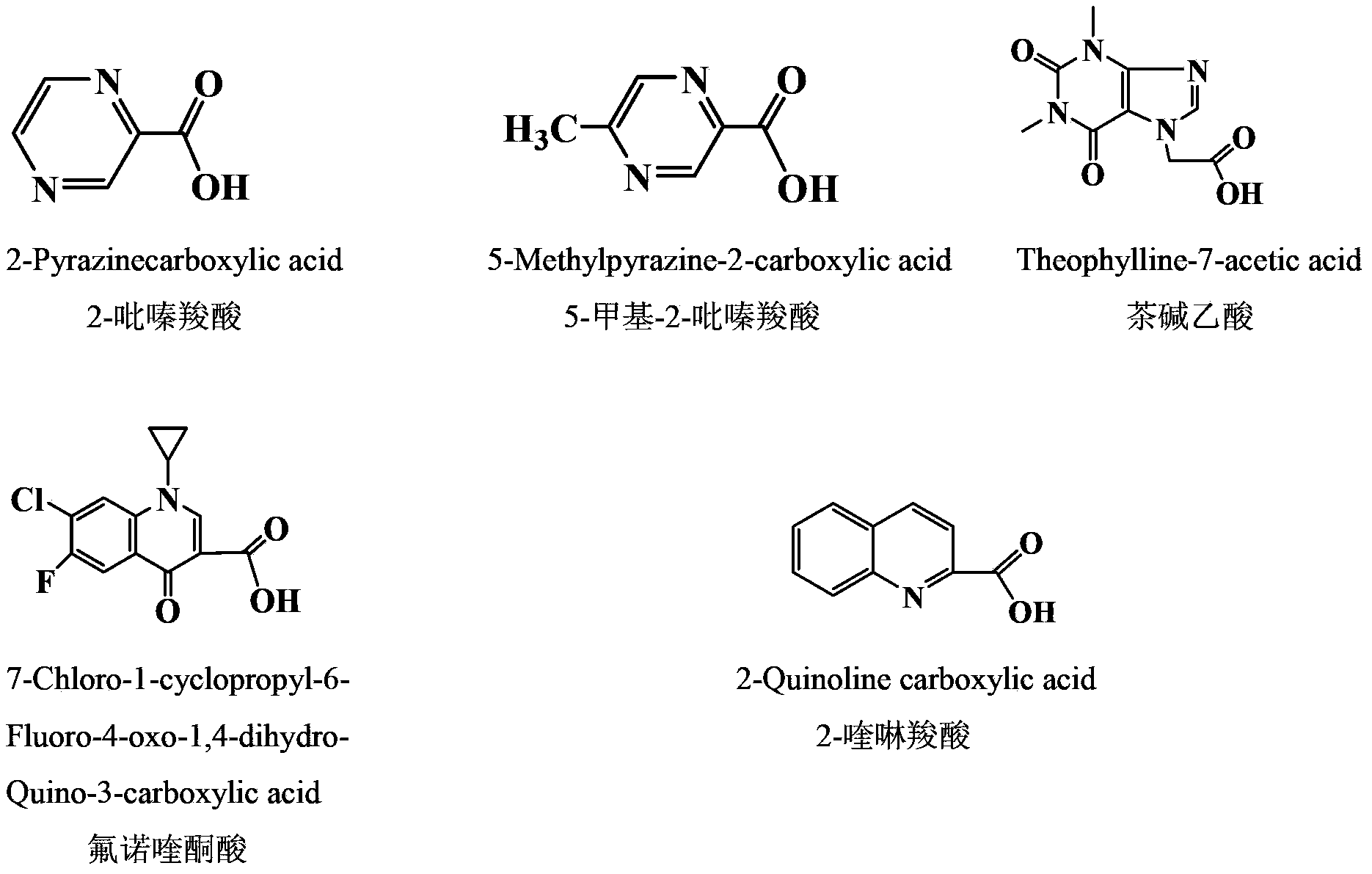
[0037] (1) Synthesis of 4-O-(2-pyrazinecarboxylic acid-1)-4-deoxy-4'-desmethyl epipodophyllotoxin: Weigh 400mg4'-desmethyl epipodophyllotoxin and 124mg2 -Pyrazinecarboxylic acid in a plate, vacuum-dried at 45°C for 2h; under the protection of nitrogen, add the dried 4'-desmethyl epipodophyllotoxin, 2-pyrazinecarboxylic acid and 208mg of dicyclohexylcarbodiimide Add 10 mL of dried dichloromethane to the four-neck flask, stir for 6 minutes, then add 40 mg of 4-dimethylaminopyridine to the reaction system, and react at room temperature for 24 hours at 25°C; after the reaction, filter the reaction solution with filter paper , remove the insoluble matter, add 100mL deionized water, keep the organic phase, repeat twice, then back-extract the aqueous phase obtained in the previous step with dichloromethane, combine the organic layers, dry overnight w...
Embodiment 2
[0043] Example 2 Synthesis and purification of 4-O-(5-methyl 2-pyrazinecarboxylic acid-1)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound 2)
[0044] (1) Synthesis of 4-O-(5-methyl-2-pyrazinecarboxylic acid-1)-4-deoxy-4'-desmethyl epipodophyllotoxin: Weigh 400mg of 4'-desmethyl epipodophyllotoxin Podophyllotoxin and 138mg of 5-methyl-2-pyrazinecarboxylic acid were placed in a plate, and vacuum-dried at 45°C for 2h; -Pyrazinecarboxylic acid and 208mg of dicyclohexylcarbodiimide were added to a four-neck flask, then 10mL of dry dichloromethane was added, and the reaction was stirred for 6min, then 40mg of 4-dimethylaminopyridine was added to the reaction system, and the mixture was heated at 25°C React for 24 hours; after the reaction is completed, filter the reaction solution with filter paper to remove insoluble matter, add 100mL deionized water, keep the organic phase, repeat twice, then back-extract the aqueous phase obtained in the previous step with dichloromethane, and ...
Embodiment 3
[0050] Example 3 Synthesis and purification of 4-O-(theophylline acetate-1)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound 3)
[0051] (1) Synthesis of 4-O-(theophylline acetic acid-1)-4-deoxy-4'-desmethyl epipodophyllotoxin: Weigh 400mg 4'-desmethyl epipodophyllotoxin and 238mg theophylline acetic acid respectively In a plate, vacuum-dry at 45°C for 2 hours; under the protection of nitrogen, add the dried 4'-desmethyl epipodophyllotoxin, theophylline acetic acid and 208 mg of dicyclohexylcarbodiimide into a four-necked bottle, and then add 5 mL Dried dichloromethane was stirred and reacted for 6 minutes, 40 mg of 4-dimethylaminopyridine was added to the reaction system, and reacted at 25° C. for 24 hours. After the reaction is completed, filter the reaction solution with filter paper to remove insoluble matter, add 100mL of deionized water, keep the organic phase, repeat twice, then use dichloromethane to back-extract the aqueous phase obtained in the previous step, and co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com