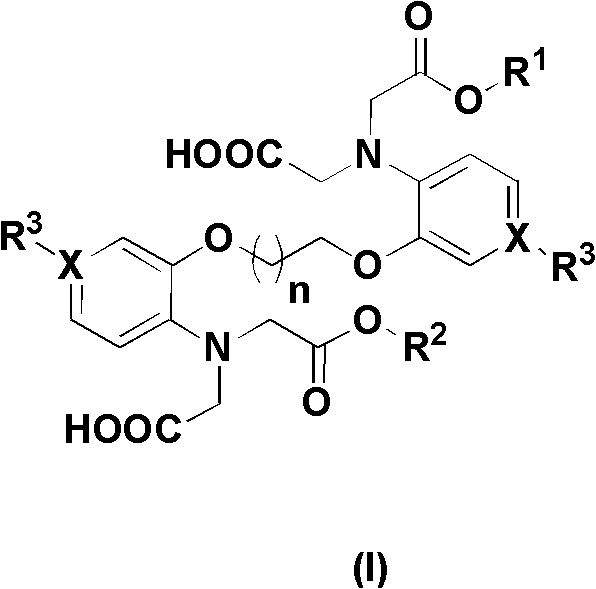
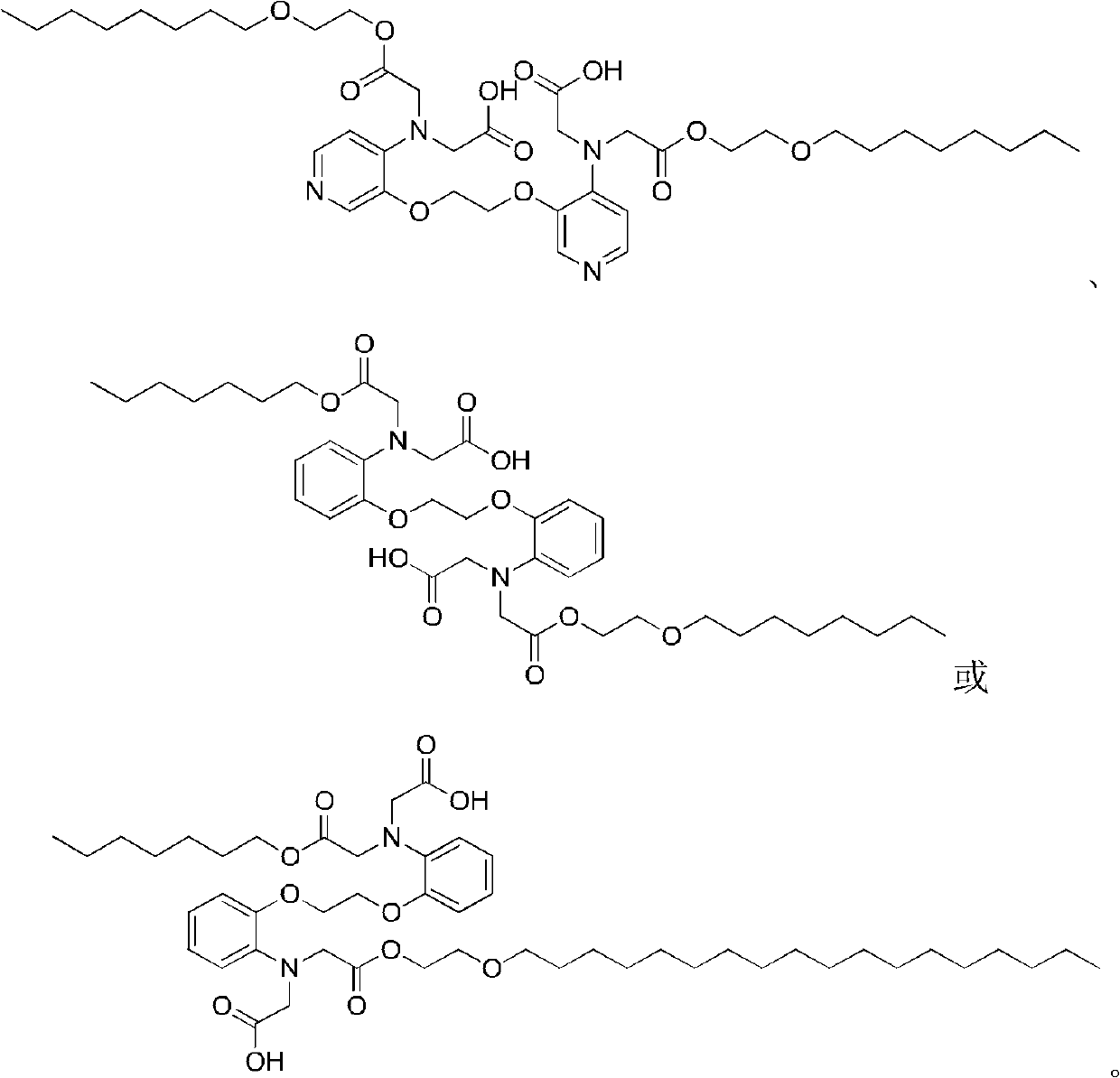
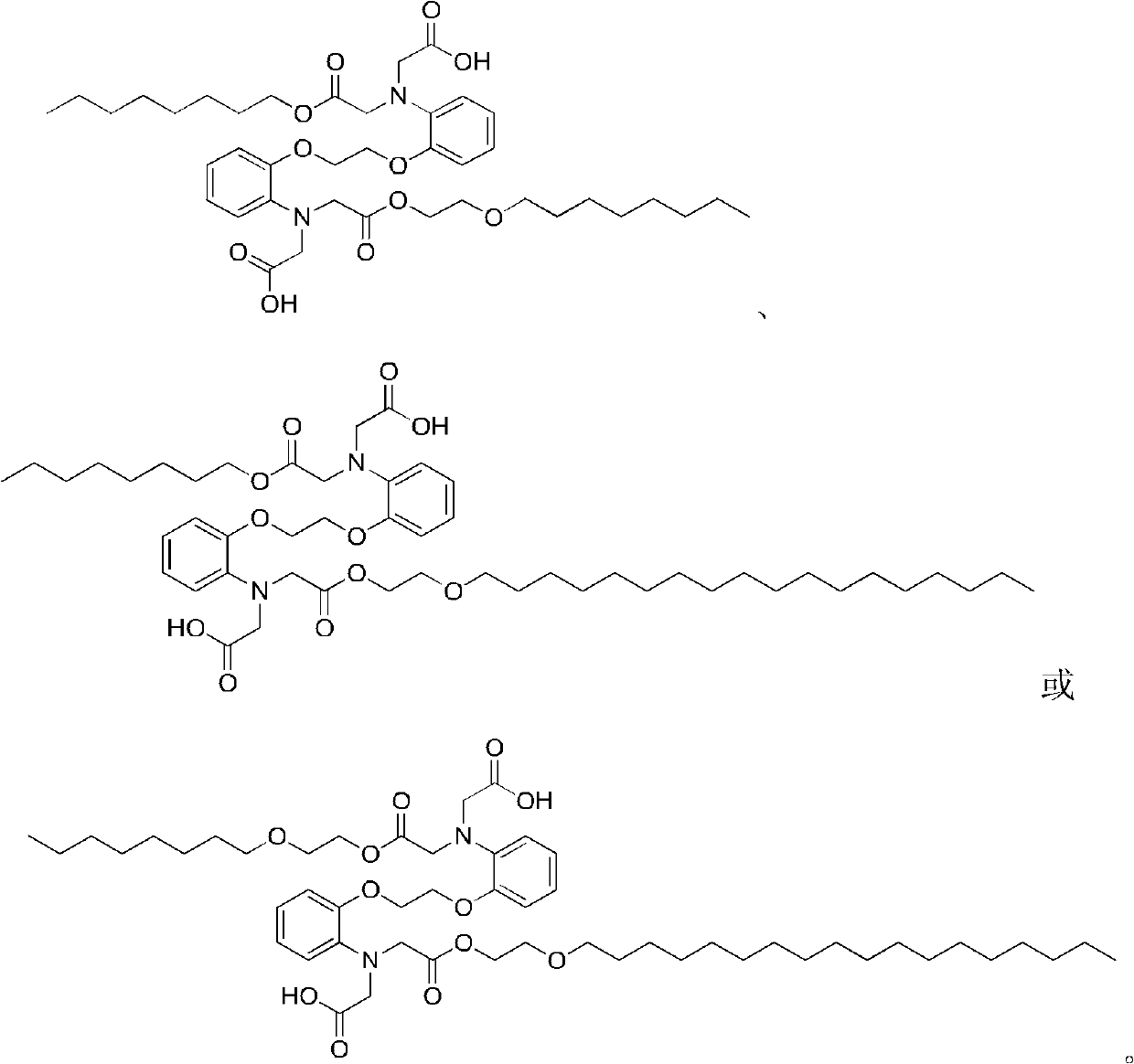
Chelate with functions of protecting nerve cells
A compound and selected technology can be used in the preparation of organic compounds, nervous system diseases, medical preparations containing active ingredients, etc., which can solve the problems of complex brain injury mechanism and affecting the prognosis of stroke.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 compound 1
[0036]
[0037] first step
[0038] Using a known method, dissolve o-nitrophenol (45g, 323.5mmol) in N, N'-dimethylformamide (500ml), add 1,2-dibromoethane (30.4g, 161.7mmol), Sodium hydroxide (19.4g, 485.3mmol), reflux reaction for 24h, TLC traced the reaction to be complete, filtered while hot, poured the filtrate into water (2000ml) while stirring, stood at room temperature for 2h, filtered, washed the filter cake with water, and dried 1-a (49.2 g, pale yellow solid) was obtained, yield: 50%.
[0039] MS m / z(ESI): 305.3[M+1]
[0040] 1 H-NMR (400 MHz, Chloroform) δ 8.29-8.21 (m, 1H), 7.81-7.68 (m, 2H), 6.88-6.80 (m, 1H), 4.50 (s, 2H).
[0041] second step
[0042] Using a known method, dissolve 1-a (40g, 131.5mmol) in ethanol (200ml), add 10% wet base palladium carbon (10g), feed hydrogen, react at room temperature overnight, follow the reaction by thin layer chromatography, filter , the filtrate was concentrated ...
Embodiment 2
[0069] The preparation of embodiment 2 compound 2
[0070]
[0071] The first to seven steps are the same as the first to seven steps of embodiment 1;
[0072] eighth step
[0073] Using a known method, dissolve 1-g (700mg, 1.3mmol) in N-methylpyrrolidone (5ml), add n-octyl-monoxyethylene (244mg, 1.4mmol), react overnight at 70°C, and thin layer Chromatography traced the complete reaction, added saturated sodium bicarbonate solution to adjust pH ≈ 8, extracted impurities with dichloromethane, adjusted pH ≈ 3 with 2N hydrochloric acid solution in the aqueous phase, filtered, washed the filter cake with water, and dried to obtain 2 (541.6mg, off-white solid), yield: 57%.
[0074] MS m / z(ESI):731.9[M+1]
[0075] 1 H-NMR (400MHz, Chloroform) δ6.87-6.75(m, 4H), 6.75-6.63(m, 4H), 5.10(s, 1H), 4.69-4.58(m, 3H), 4.49(s, 4H) , 4.38(s, 1H), 4.14(d, 6H), 3.88(s, 1H), 3.61(d, 2H), 3.36-3.26(m, 2H), 1.68-1.38(m, 8H), 1.38-1.14 (m, 14H), 0.98-0.81 (m, 6H).
Embodiment 3
[0076] The preparation of embodiment 3 compound 3
[0077]
[0078] The first to seven steps are the same as the first to seven steps of embodiment 1;
[0079] eighth step
[0080] Using a known method, dissolve 1-g (700mg, 1.3mmol) in N-methylpyrrolidone (5ml), add 2-octadecyloxyethanol (440.4mg, 1.4mmol), react at 70°C overnight, thin The reaction was followed by layer chromatography, adding saturated sodium bicarbonate solution to adjust pH ≈ 8, extracting impurities with dichloromethane, adjusting pH ≈ 3 with 2N hydrochloric acid solution in the aqueous phase, filtering, washing the filter cake with water, and drying to obtain 3 (622.9mg, analogue White solid), yield: 55%.
[0081] MS m / z(ESI): 872.2[M+1]
[0082] 1 H-NMR (400MHz, Chloroform) δ6.89-6.76(m, 4H), 6.76-6.64(m, 4H), 5.04(s, 1H), 4.74(s, 1H), 4.56(s, 1H), 4.50 (d, 5H), 4.28(s, 1H), 4.20(d, 3H), 4.13(t, 2H), 4.04(s, 1H), 3.70(s, 1H), 3.63(t, 2H), 3.33( t, 2H), 1.69-1.18 (m, 44H), 0.97-0.82 (m, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com