Solid-phase synthesis process of angiotensinamide as well as intermediate and application thereof
A technology of blood-increasing hormone and condensation reaction, which can be used in medical preparations containing active ingredients, drug combinations, cardiovascular system diseases, etc., and can solve problems such as difficulty in complete dissociation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0116] 9. Preparation of vasopressin
[0117]
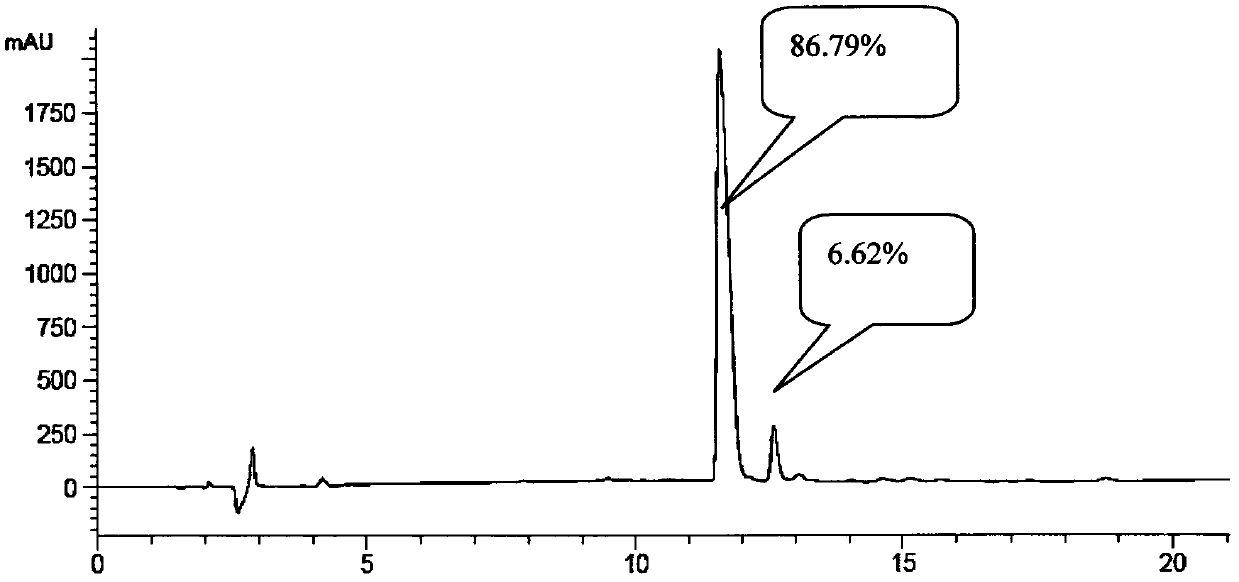
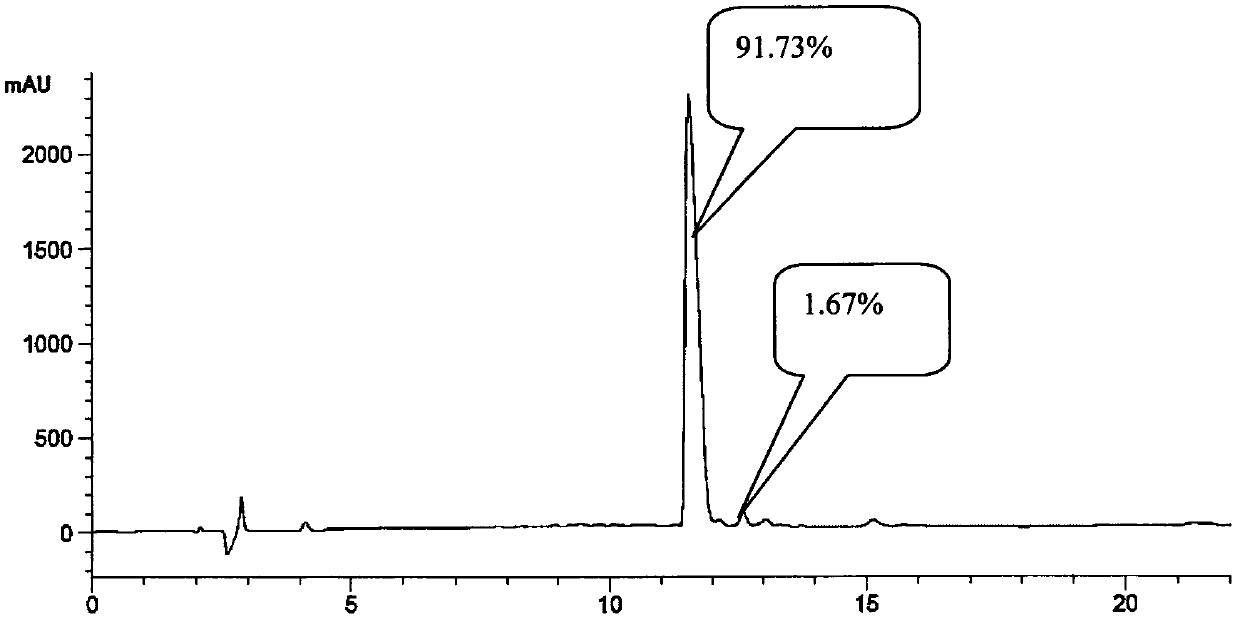
[0118] Adding protected vasopressin to peptide cutting reagent TFA:TIS:H 2 In O, react for 2-3 hours, filter with suction, concentrate the filtrate by distillation under reduced pressure, add ice-cold methyl tert-butyl ether to precipitate, and collect the crude vasopressin by centrifugation. The crude peptide was purified by reverse-phase high-performance liquid chromatography to obtain a pure white product.
Embodiment 1
[0120] Embodiment 1: the preparation of Fmoc-Phe-Wang resin
[0121] Weigh 50g of Wang resin (100-200, 1.23mmol / g), put it into a sand core reactor, wash it once with 500mL DMF, drain it, then use 500mL DCM to fully swell the resin, and drain it.
[0122] Add Fmoc-Phe-OH (MW: 387.4, 3 times the moles of Wang resin) 71.5g, HOBt (MW: 135.13, 3 times the moles of Wang resin) 24.9g, DIC (MW: 126.2, 3 times the moles of Wang resin) 3 times) 28.9mL, 300mL DMF, 100mL DCM, use the nitrogen flow to fully and uniformly contact with the peptide resin, and use the volatilization and endothermic of DCM to quickly cool down the entire reaction system to 16 ° C, slowly add DMAP (MW: 122, Wang resin mole 0.2 times the number) 1.5 g, and the mixture was stirred at 26°C for 18 hours. Vacuum-dried, washed twice with DMF, and vacuum-dried. Add Pyridine (MW: 79.1, 10 times the number of moles of Wang resin) 49mL, Ac 2 O (MW: 102.09, 10 times the number of moles of Wang resin) 58mL, 500mL DCM, t...
Embodiment 2
[0123] Embodiment 2: the preparation of Fmoc-Pro-Phe-Wang resin
[0124] Add 500mL 20% Pi p / DMF solution, stir at 25°C for 10 minutes, vacuum dry, then add 500mL 20% Pi p / DMF solution, stir at 25°C for 20 minutes, vacuum dry, wash once with DMF, MeOH Twice, washed once with DMF, once with DCM, and dried in vacuo. Add 40.5g of Fmoc-Pro-OH (MW: 337.4, twice the molar number of Fmoc-Phe-Wang resin), 16.2g of HOBt (MW: 135.13, twice the molar number of Fmoc-Phe-Wang resin), HBTU (MW : 379.25, 2 times the number of moles of Fmoc-Phe-Wang resin) 45.5g, 350mL DMF, 100mL DCM, use the nitrogen flow to fully and evenly contact the peptide resin, and use the volatilization and heat absorption of DCM to quickly cool the entire reaction system to 15 °C , DIPEA (MW: 129.24, 2 times the mole number of Fmoc-Phe-Wang resin) 31.4mL was added dropwise, and the mixture was stirred at 25°C for 2.5 hours. Chlorobenzoquinone was tested negative, dried in vacuo, washed once with MeOH, DMF Wash twic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com