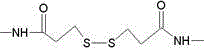A quality control product to replace positive blood of patients
A quality control product and positive technology, applied in the field of immunology, can solve problems such as difficult inactivation, difficult standardization, and unstable quality, and achieve the effects of simple production and preparation, reducing detection errors, and avoiding infectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Use SPDP cross-linking agent (purchased from ThermoScientific, product number 21857) to prepare mouse anti-HSV-IIMcAb and healthy non-HSV-II-infected human IgG heterologous cross-linked product as a substitute for HSV-II antibody in positive blood of patients , the process is as follows:
[0044] (1) Dissolve 5 mg mouse anti-HSV-IIMcAb in cross-linking buffer (0.1M potassium phosphate, 0.1M NaCl, pH7.5), stir and add 50 μl SPDP cross-linking agent (3.2 mg / ml, dissolved in absolute ethanol Middle), after reacting at room temperature for 2 hours, put it into a dialysis bag, dialyze with reducing buffer (0.1M sodium acetate, 0.1M NaCl, pH4.5), and change the solution four times;
[0045] (2) Dissolve 5 mg of healthy human IgG uninfected with HSV-II in the cross-linking buffer, stir and add 50 μl SPDP cross-linking agent, react at room temperature for 2 hours, put it into a dialysis bag, and dialyze with the cross-linking buffer, Change the liquid four times;
[0046] (3)...
Embodiment 2
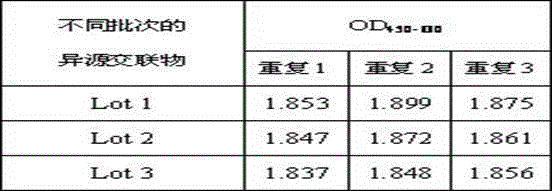
[0052] Example 2 Mouse anti-HSV-IIMcAb and healthy non-infected HSV-II human IgG heterologous cross-linked substance substitution, quality control stability and cryopreservation stability experiments
[0053] The mouse anti-HSV-IIMcAb prepared in Example 1 and the heterologous cross-linked product of healthy human IgG not infected with HSV-II were used as a substitute for the HSV-II antibody in the patient's positive blood to carry out experiments.
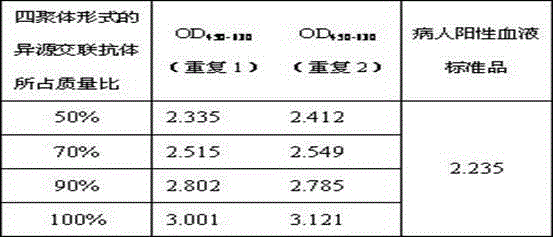
[0054] (1) Preparation of quality control products. The heterologous cross-linked products with tetrameric heterologous cross-linked antibodies accounted for 50%, 70%, 90% and 100% of the mass ratio in the prepared heterologous cross-linked products were sequentially prepared as patient Surrogates for HSV-II antibodies in positive blood. When the heterologous cross-linked antibody in tetrameric form accounts for 50%, 70% or 90% of the mass ratio of the prepared heterologous cross-linked product, the remaining 50%, 30% or 10% hete...
Embodiment 3
[0074] Example 3 Mouse anti-HSV-IMcAb and healthy non-infected HSV-1 human IgG heterologous cross-linked substance substitution experiment
[0075] Using SPDP cross-linking agent to prepare mouse anti-HSV-IMcAb and healthy non-infected HSV-I human IgG heterologous cross-linked product as a surrogate for HSV-I antibody in the patient's positive blood, in addition to using mouse anti-HSV- Except that the IMcAb replaced the mouse anti-HSV-IIMcAb, the specific process was the same as in Example 1.
[0076] Experiments were carried out using the mouse anti-HSV-IMcAb prepared in Example 3 and the heterologous cross-linked product of healthy human IgG not infected with HSV-1 as a substitute for the HSV-1 antibody in the patient's positive blood. The heterologous cross-linked products in which the mass ratio of tetrameric heterologous cross-linked antibody in the prepared heterologous cross-linked product is 50%, 70%, 90% and 100% are sequentially prepared. When the heterologous cros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com