Miltenberger blood group antibody detection test strip and detection method thereof
A detection method and antibody detection technology, which is applied in the field of medical detection, can solve the problems of antigen depletion, shortage of antigen reagents and antibody reagents, antibody detection restrictions, etc., and achieve the effect of ensuring clinical safety, scientific blood transfusion, and quick and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
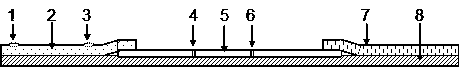
[0024] see figure 1 , a Miltenberger antibody detection immunofluorescence chromatography test strip, comprising a reaction membrane 5, a sample pad 2, an absorption pad 7 and a liner 8, the reaction membrane 5 is located on the liner 8, and the reaction membrane 5 A detection belt 4 and a quality control belt 6 are fixed, and the sample pad 2 and the absorbent pad 7 partially overlap the two sides of the reaction membrane 5 respectively, and are located on the reaction membrane 5 and the backing plate 8, The sample pad 2 includes a first sample application point 1 and a second sample application point 3, the first sample application point 1 is arranged on the side away from the sample pad 2 overlapping with the reaction membrane 5, the The second sample adding point 3 is set close to the side where the sample pad 2 overlaps with the reaction membrane 5 .
[0025] Preferably, the material of the reaction membrane 5 is one of nitrocellulose membrane, cellulose acetate membrane...
Embodiment 2
[0027] Provide a kind of detection method that the test strip described in embodiment one is used for Miltenberger blood type antibody screening:
[0028] (1) Preparation of test strips:
[0029] 1) Select Millipore HF 180 backing membrane as the reaction membrane, cut it into 30×1.8 cm size for use, adjust the concentration of human IgG and human IgM to 0.5 mg / mL with 0.01 M PBS with a pH value of 7.2, Add Tween-20 with a volume fraction of 1%, and spray it on the surface of the NC membrane as a quality control strip using a film-scribing device, and the film-scribing volume is 0.5 μL / cm. Streptavidin was adjusted to a concentration of 0.1 mg / mL using 0.15 M PBS with a pH value of 7.2, and was sprayed on the surface of the NC membrane as a detection zone using a film-drawing device, with an interval of 5 mm between each line, and the film was drawn Immediately after the end, put it in a 37°C oven to dry overnight, and store it at room temperature for later use;
[0030] 2) ...
Embodiment 3
[0048] Provide a kind of detection method that the test strip described in embodiment one is used for the specific identification of Miltenberger blood group antibody:
[0049] (1) Preparation of test strips:
[0050] 1) Select Millipore HF 180 backing membrane as the reaction membrane, cut it into 30×1.8 cm size for use, adjust the concentration of human IgG and human IgM to 0.5 mg / mL with 0.01 M PBS with a pH value of 7.2, Add Tween-20 with a volume fraction of 1%, and spray it on the surface of the NC membrane as a quality control strip 6 using a film-scribing instrument, and the film-scribing volume is 0.5 μL / cm. Streptavidin was adjusted to a concentration of 0.1 mg / mL using 0.15 M PBS with a pH value of 7.2, and was sprayed on the surface of the NC membrane as a detection zone using a film-drawing device, with an interval of 5 mm between each line, and the film was drawn Immediately after the end, put it in a 37°C oven to dry overnight, and store it at room temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com