A kind of method for preparing primaquine phosphate
A technology of primaquine phosphate and aminoquinoline, which is applied in organic chemistry and other fields, and can solve problems such as the limitation of intermediate yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Reaction of methyl vinyl ketone with nitromethane
example 1
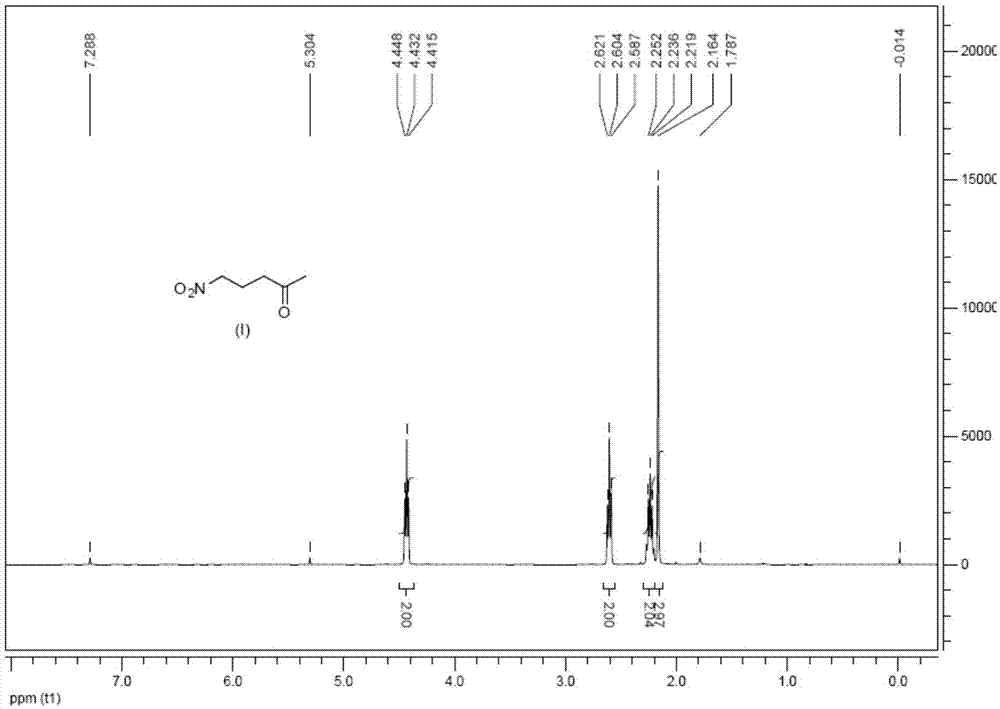
[0052] Add 8.0 ml (0.1 mol) of methyl vinyl ketone, 32 ml (0.6 mol) of nitromethane and 4.0 g of basic alumina-supported potassium fluoride catalyst into 80 ml of ethanol and stir at room temperature for 24 hours. After the reaction was finished, the insolubles were removed by suction filtration, ethanol and excess nitromethane were distilled off under normal pressure, and the 5-nitropentan-2-one (compound of formula I) distilled under reduced pressure was 11.2 g of light yellow oily matter. The rate is 87%. The proton NMR spectrum of the product (see the attached figure 1 ): 1 H NMR (400MHz, CDCl 3 )δ=4.42(t,J=6.6Hz,2H),2.60(t,J=6.8Hz,2H),2.26-2.19(m,2H),2.15(s,3H).
example 2
[0054] The scale of the reaction in Example 1 was expanded to 100 ml of methyl vinyl ketone, and other materials were expanded in proportion. After 24 hours of reaction, 119 grams of 5-nitropentan-2-one were distilled under reduced pressure, with a yield of 92.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com