A kind of method for preparing 2-chloro-4-nitroimidazole
A technology of nitroimidazole and clomidazole, which is applied in the direction of organic chemistry, can solve the problems of easy explosion accidents, many by-products, and difficult industrialization, and achieve the effects of easy industrial production, high product quality, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
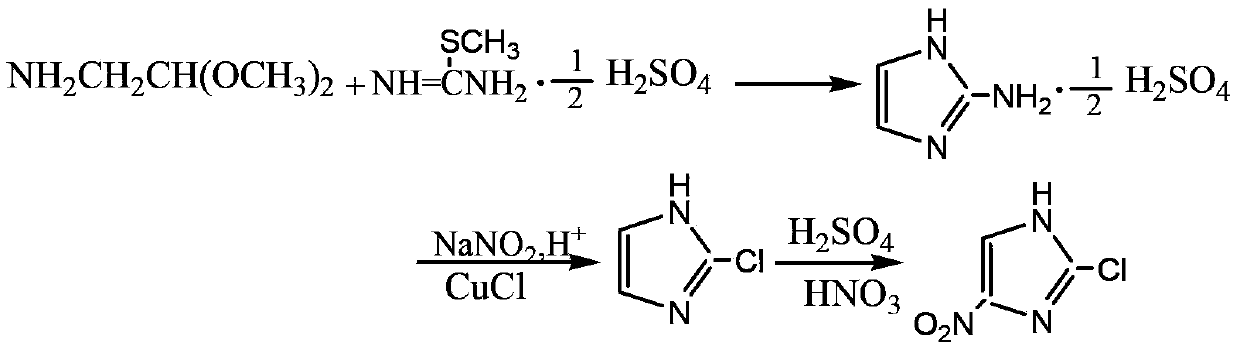
[0021] Add 9.94g of aminoacetaldehyde diethyl acetal (0.074mol), 20.6g of S-methylisothiourea sulfate (0.074mol) and 16.5ml of water into the three-necked flask successively, then raise the temperature to 110°C, and stir the reaction for 1.5h. Stop responding. Evaporate the solvent, then add 13.0g of 37% hydrochloric acid (mass percent concentration, the same as in the following examples) to the reaction solution, stir at 65°C for 1 hour, add 50ml of water, evaporate the solvent, add 30ml of acetone, and precipitate light yellow crystals. After filtration, the crude product was recrystallized with 30ml of ethanol-water to obtain 15.90g of off-white powdery solid 2-aminoimidazole sulfate, yield 81.5%, mp271.2~273.0℃.
[0022] Add 15g of 37% hydrochloric acid and 10.56g of 2-aminoimidazole sulfate (0.04mol) into a three-necked flask, and dropwise add 10.8g of 25.9% sodium nitrite solution (mass percentage concentration, the same as the following examples) (0.04mol) under ice-wat...
Embodiment 2
[0025] Add 7.8g of aminoacetaldehyde dimethyl acetal (0.074mmol), 20.6g of S-methylisothiouronium sulfate (0.074mmol) and 16.5ml of water into the three-necked flask successively, then raise the temperature to 110°C, and stir for 1.5h. Stop responding. Evaporate the solvent, then add 13.0g of 37% hydrochloric acid (mass percent concentration, the same as in the following examples) to the reaction solution, stir at 65°C for 1 hour, add 50ml of water, evaporate the solvent, add 30ml of acetone, and precipitate light yellow crystals. After filtration, the crude product was recrystallized with 30ml of ethanol-water to obtain 15.31g of off-white powdery solid 2-aminoimidazole sulfate, yield 78.5%, mp271.2~273.0℃.
[0026] Add 15g37% hydrochloric acid and 3.3g2-aminoimidazole (0.04mol) into the three-necked flask, add dropwise 10.8g25.9% sodium nitrite solution (mass percentage concentration, the same as the following examples) (0.04mol) under ice-water bath, maintain The reaction ...
Embodiment 3
[0029] With the method of embodiment 1, the difference is that the reaction molar ratio of aminoacetaldehyde diethyl acetal and S-methylisothiouronium sulfate is 1:5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com