Preparation method of nifuratel
A nifuratel and crude technology is applied in the refining field of nifuratel, can solve the problems of high cost of liquid chromatography, unsuitable for large-scale production, expensive and difficult to obtain, etc., and achieves low price, low cost, environmental protection and the like. friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
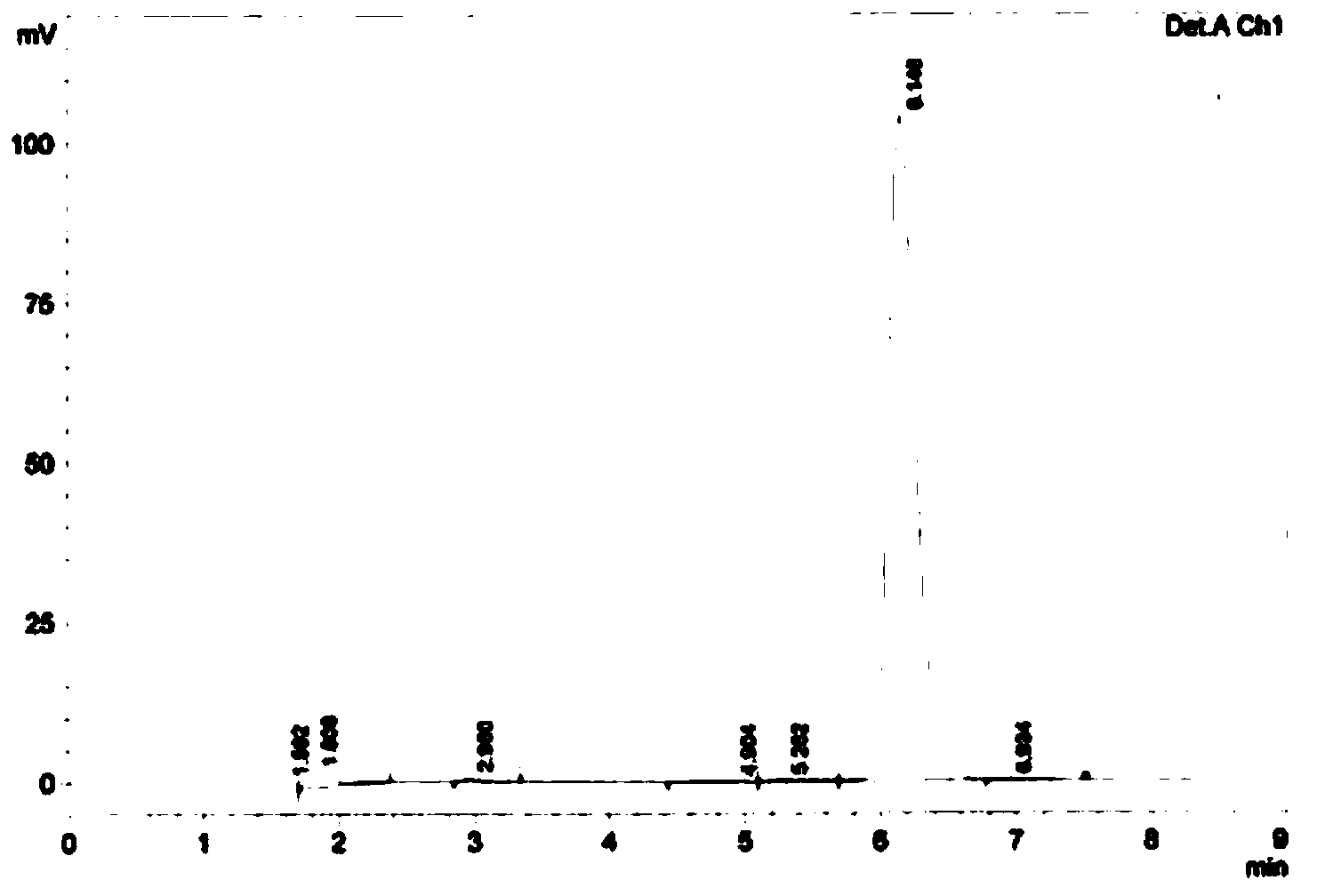
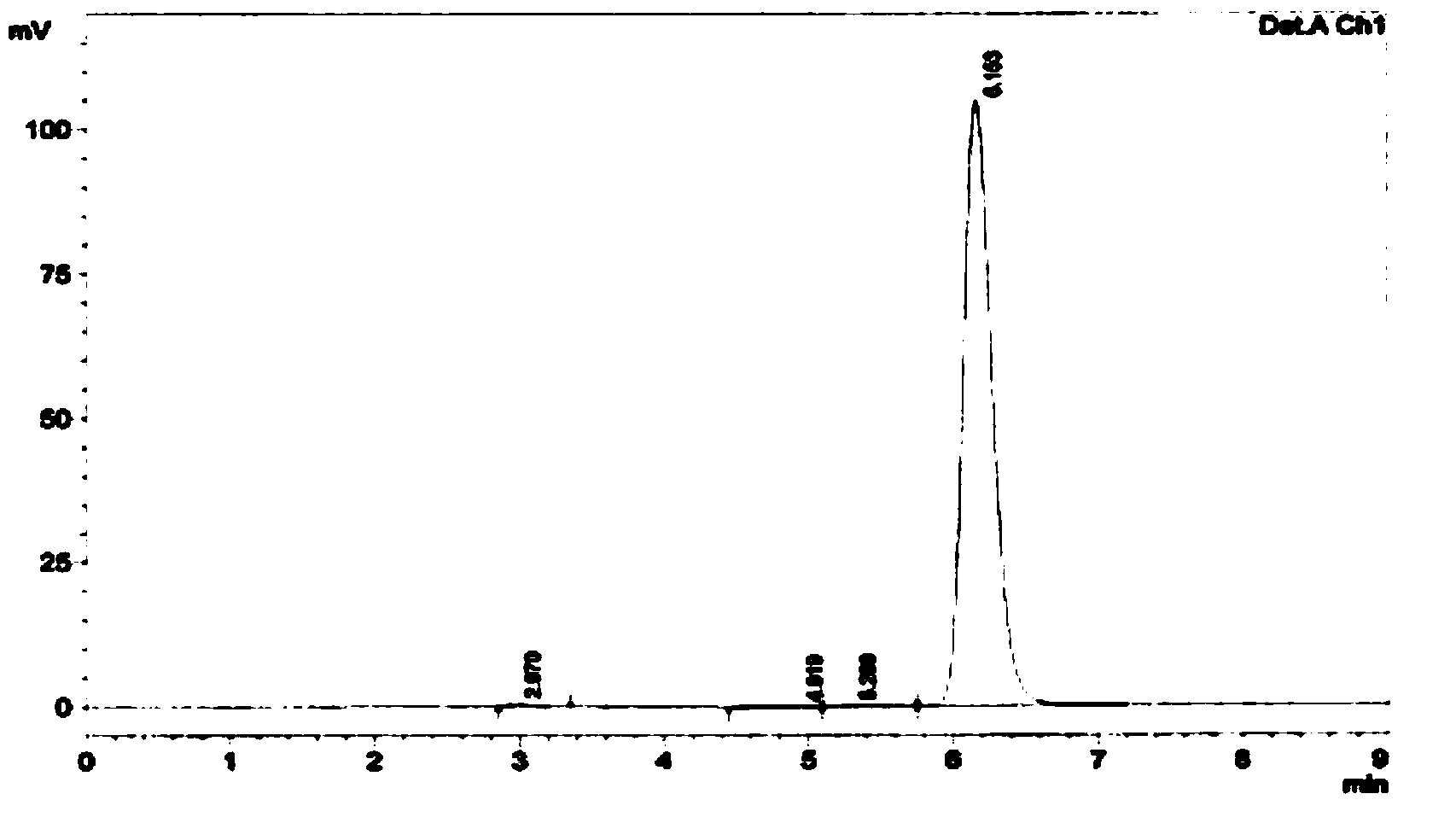
Image
Examples
Embodiment Construction
[0034] The present invention will be described in detail below with reference to the accompanying drawings and examples.
[0035] Preparation Example
[0036] one. Crude Nifuratel
[0037] This embodiment comprises three steps of cyclization, hydrolysis and condensation reaction:
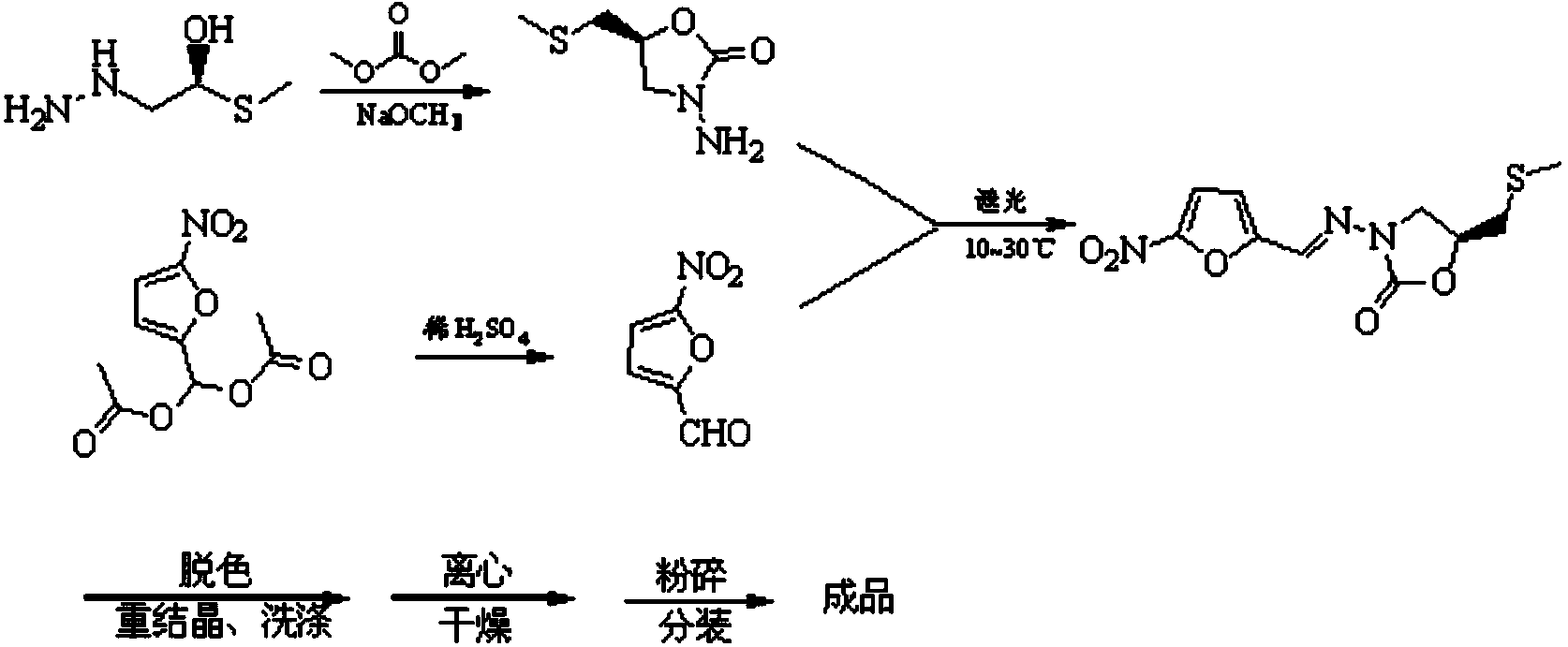
[0038] The first step: cyclization reaction to prepare 3-amino 5-[methylthio(methyl)]-2-oxazolidinone
[0039] The reaction scheme is as follows:
[0040]
[0041] Put 48kg of 1-hydrazino-3-methylthio-2-propanol into the cyclization reaction tank, start stirring, then inhale 34kg of dimethyl carbonate into the cyclization reaction tank, and continue to stir for 10min; 19kg of sodium methoxide-methanol The solution (wherein the content of sodium methoxide is 27.5wt%) is sucked into the dripping tank, the dripping valve is opened, and it is dripped into the ring-closure reaction tank while stirring, and the dripping is completed in about 30 minutes; the cooling water of the sheet condenser is o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com