Method for synthesizing TATB (triamino trinitrobenzene) by normal pressure phase-transfer catalysis and amination
A phase-transfer catalyst and phase-transfer technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds, etc., can solve the problems of unfavorable large-scale industrial production, low solubility, difficult to purchase, etc., and achieve a large-scale industrialization Large-scale production, simple reaction operation, and the effect of solving the problem of chlorine content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
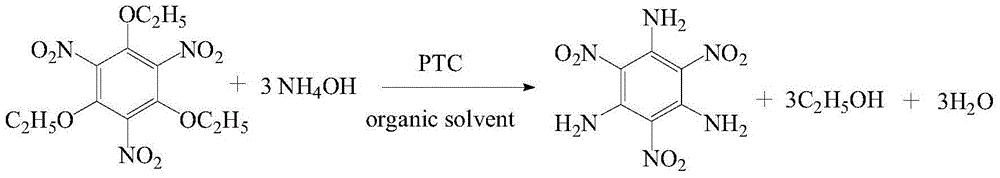
[0027] Add 2.070g 1,3,5-triethoxy-2,4,6-trinitrobenzene and 15ml xylene into a four-necked flask, heat to 50℃ in a water bath with stirring, and add 15ml ammonia water (weight percentage 25-28%) reflux the reaction for 2h. After the reaction, the reaction solution is cooled to room temperature, filtered to obtain TATB, washed with ethanol for 2-3 times and dried to obtain 0.093g of TATB product, with a yield of 6%. The purity of TATB was 98.32% by high performance liquid chromatography, and the exothermic decomposition temperature by DSC was 386.12%.
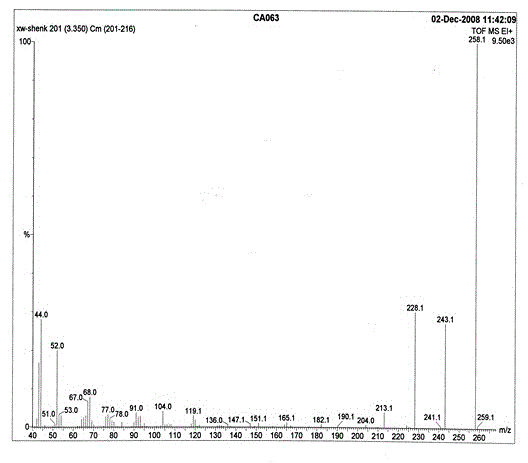
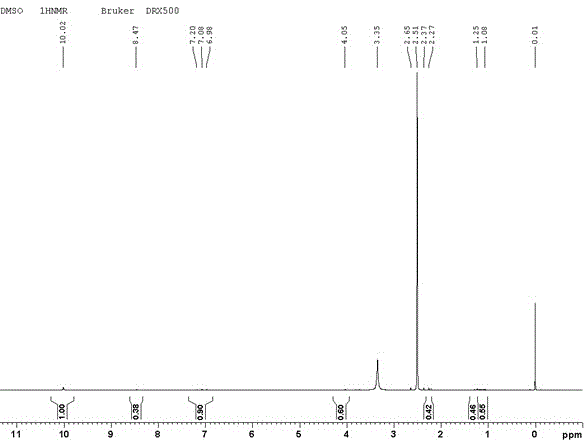
[0028] Spectral data: MS(m / z): 258.1(M + ); 1 H-NMR(DMSO-d6), δ: 3.35(m, 6H, -NH 2 ); FT-IR(KBr), ν(cm -1 ): 3320, 3216, 1604, 1571, 1444, 1217, 1166, 781, 727, 694.
Embodiment 2
[0030] Add 2.070g 1,3,5-triethoxy-2,4,6-trinitrobenzene, 15ml xylene and 0.040g cetyltrimethylammonium bromide into a four-neck flask, and heat in a water bath while stirring At 50°C, 15ml ammonia water (25-28% by weight) was added and refluxed for 2h. After the reaction, the reaction solution was cooled to room temperature, filtered to obtain TATB, washed with ethanol for 2-3 times and dried to obtain TATB product 0.805. The purity of TATB was 98% by high performance liquid chromatography, the yield was 52.00%, and the exothermic decomposition temperature by DSC was 378.84%.
Embodiment 3
[0032] Add 2.070g 1,3,5-triethoxy-2,4,6-trinitrobenzene, 15ml xylene and 0.40g β-cyclodextrin into a four-necked flask, stir and heat in a water bath at 50℃, add 15ml ammonia (The weight percentage is 25-28%) The reaction was refluxed for 2h. After the reaction, the reaction solution was cooled to room temperature, filtered to obtain TATB, washed with acetone for 2-3 times and dried to obtain 0.09g of TATB product, with a yield of 5.8%. The purity of TATB was 98.15% by high performance liquid chromatography, and the exothermic decomposition temperature by DSC was 378.84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com