L-carnosine preparation method
A technology of carnosine and reaction, applied in the field of preparation of L-carnosine, can solve the problems of difficult large-scale production, few reaction steps, low yield, etc., and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
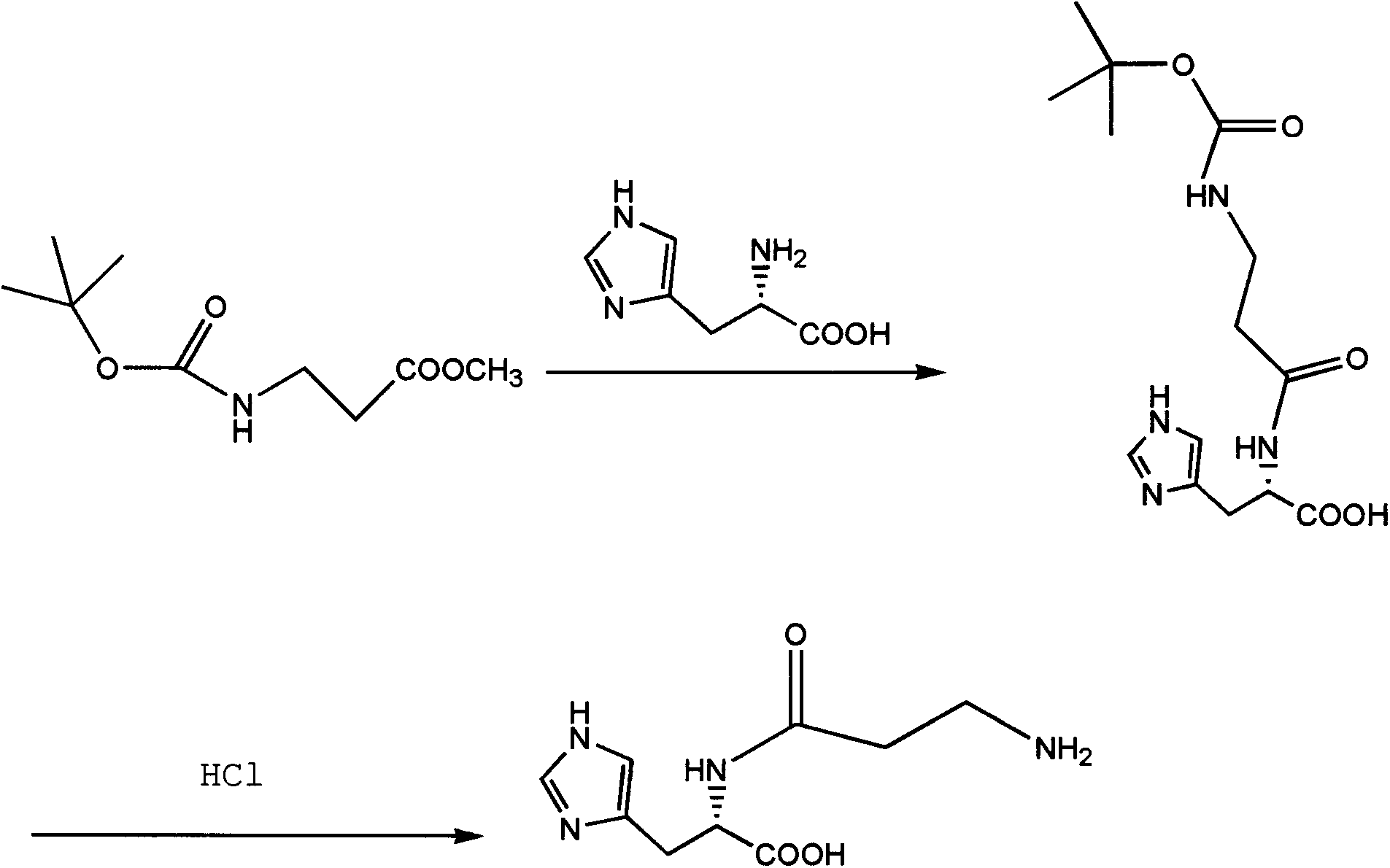
[0017] Preparation of N-(N-BOC-propionyl)-L-histidine
[0018] Add 11-12Kg of L-histidine, 15.4Kg of N-BOC-β-alanine methyl ester hydrochloride and 80-100L of DMSO into the reactor, slowly add 5.2-6.5Kg of sodium ethoxide, and raise the temperature to 120-125°C. React for 5-6 hours, when the reaction is over, lower the temperature to 50°C, slowly add concentrated hydrochloric acid to adjust the pH to 2-3, then concentrate under reduced pressure to 12-15L, cool to room temperature, add 50-55L of acetone, stir in an ice bath, and precipitate coarse crystals , filtered to obtain a crude product with a weight yield of 30-32Kg.
Embodiment 2
[0020] Preparation of N-(N-BOC-propionyl)-L-histidine
[0021] Add 11-12Kg of L-histidine, 15.4Kg of N-BOC-β-alanine methyl ester hydrochloride and 80-100L of DMSO into the reactor, slowly add 1.9-2.1Kg of sodium hydride, and raise the temperature to 120-125°C. React for 5-6 hours, after the reaction is over, lower to room temperature, slowly add concentrated hydrochloric acid to adjust the pH to 2-3, then concentrate under reduced pressure to 12-15L, then cool to room temperature, add 50-55L of acetone, stir in an ice bath, and precipitate coarse crystals , filtered to obtain the crude product with a weight yield of 32-34Kg.
Embodiment 3
[0023] Preparation of L-carnosine
[0024] Add 30Kg of N-(N-BOC-propionyl)-L-histidine to the reaction kettle, add 60~70L of formic acid and stir to dissolve, 30L of 4mol / L hydrochloric acid / isopropanol solution, react at room temperature for 1.5~2.0h, use Dilute sodium bicarbonate to adjust the pH to 7-8, add 20-25L of ethanol, precipitate crystals, filter, wash with ether, and dry to obtain 26-29Kg of the product, with a total yield of 72-75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com