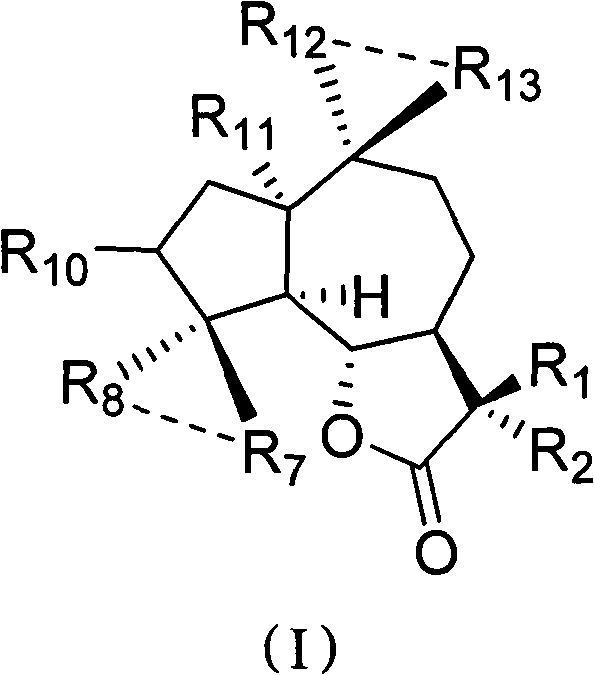
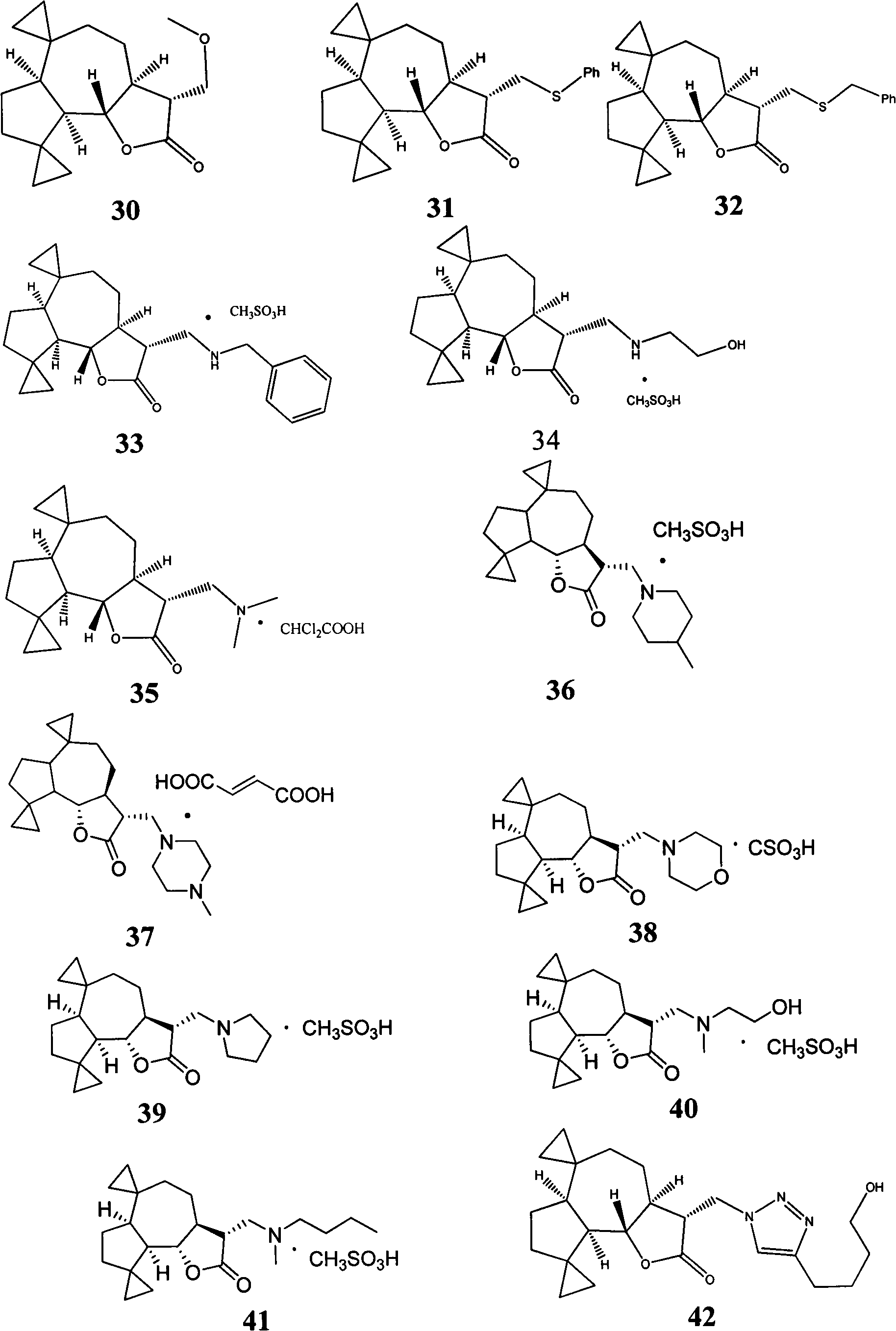
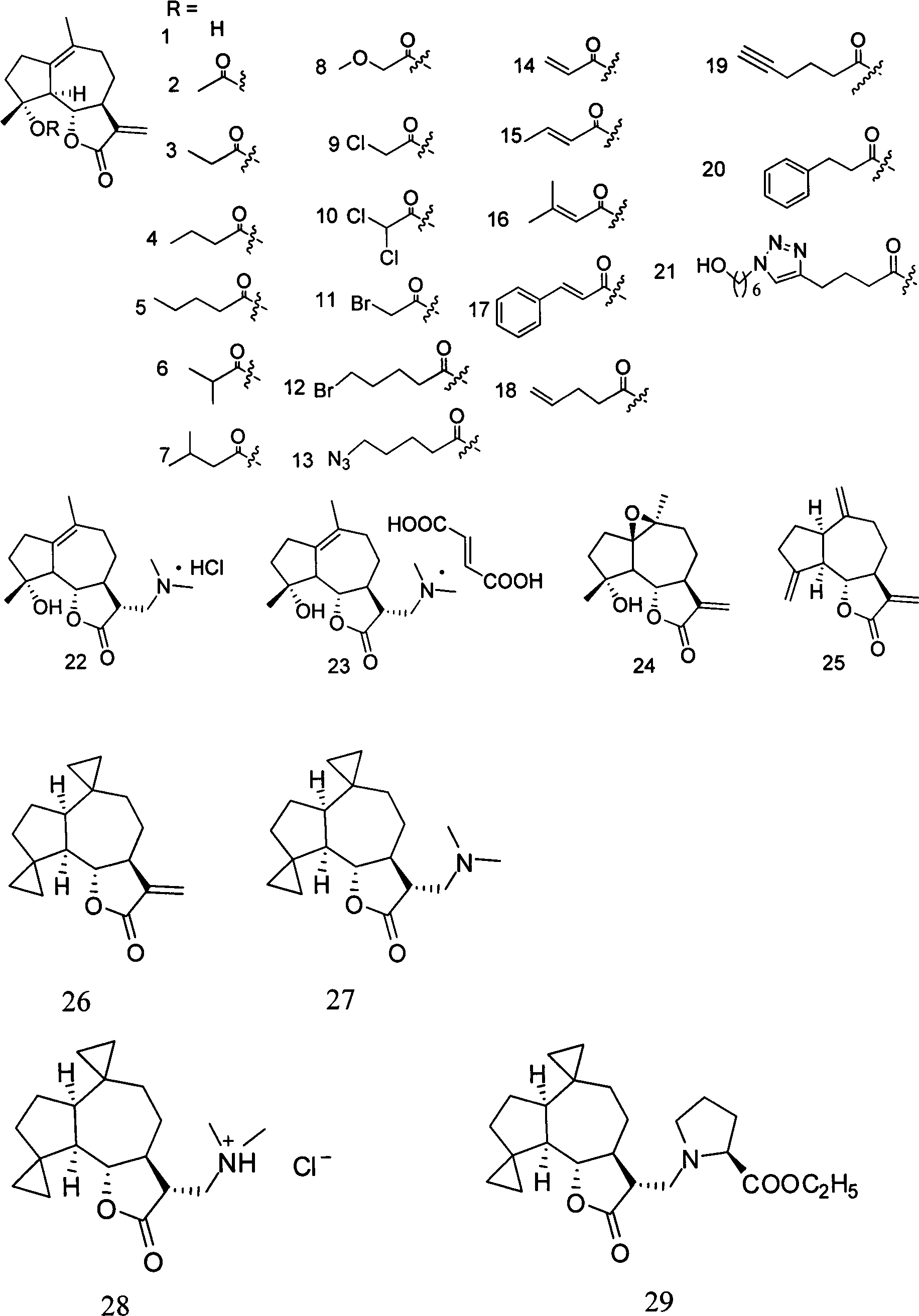Sesquiterpene lactone compound and uses of derivative thereof in preparation of drugs
A technology for preparing drugs and compounds, which is applied in the field of inhibiting cancer stem cells, treating cancer drugs, and preparing and treating rheumatoid arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation method of compound 1-50
[0029] Preparation of compound 1:
[0030] Parthenolide (50 mg, 0.2 mmol) was dissolved in 2.5 mL of dichloromethane, and p-toluenesulfonic acid (5 mg, 0.026 mmol) was added. The reaction system was left at room temperature and stirred overnight. Transfer the reaction solution to NaHCO 3 (10mL) in a saturated solution, collect the organic phase, extract the aqueous phase with a small amount of dichloromethane, combine the organic phases, and wash with Na 2 SO 4 After drying and filtering, the organic solvent was distilled under reduced pressure with a rotary evaporator, and purified on a silica gel column to obtain compound 1 (45 mg, yield 90%). 1 H NMR (CDCl 3 , 400MHz) δ6.20(d, J=3.2Hz, 1H) 5.49(d, J=3.2Hz, 1H) 3.81(t, J=10.4Hz, 1H), 2.70(d, J=10.4Hz, 1H) , 2.65-2.62(m, 2H), 2.40-2.34(m, 1H), 2.07-2.26(m, 4H), 1.73-1.86(m, 2H), 1.68(s, 3H), 1.36-1.28(m, 4H); 13 C NMR (CDCl 3, 100MHz) δ169.8, 138.7, 131.7...
Embodiment 2
[0171] Example 2: Anti-rheumatoid arthritis activity test of compound 1-50
[0172] In the field of rheumatoid arthritis drugs, there are many literatures reporting the effects of drug ingredients on the secretion of TNF-α, PGE2 and IL-1β by synoviocytes, and the data of animal experiments are used to investigate the effect of drug treatment of RA. These documents include: [1] Ju Dahong, Jia Hongwei, Wu Hao, etc., Effects of Lugua Polypeptide Injection on C II-induced Immune Arthritis Rat Serum TNF-α, IL-6 and C II Antibody Activity, Chinese Traditional Medicine Foundation Medical Journal, 2003, 9(11):17. [2] He Jinhua, Liang Qinghua, Zhang Huasheng, etc., Effect of Bizhongxiao Decoction on Plasma TNF-α in Rats with Experimental Arthritis, Journal of Hunan Medical University, 2002, 27(5): 524. [3] Huang Qingchun , Zhang Shengpeng, Xu Qiuying, Effects of Compound Salvia Miltiorrhiza on Type II Collagen-Induced Synoviocyte Secretion and Tumor Necrosis Factor in Rat Model, Moder...
Embodiment 3
[0187] Example 3: Activity test of compounds inhibiting cancer stem cells
[0188] Take fresh or frozen (acute myeloid leukemia (AML, marked as CD34 + / CD38 + ), chronic myeloid leukemia (CML, Ph+ / CD34+ / CXCR4+), chronic lymphocytic leukemia (CLL, CD133+ / CD19- / CD38-), skin cancer (CD34+), breast cancer (CD44+ / CD24- / ESA+), ovarian cancer (CD44+ / CD117+), brain tumor (CD133+), prostate cancer (CD44+ / CD24-), head and neck squamous cell carcinoma (CD44+), laryngeal cancer (CD 133+), pancreatic cancer (ESA+ / CD44+ / CD24+), retinoblastoma (ABCG2 / ALDH1), childhood hepatoblastoma (CD34+ / THY1+ / c-kit+), liver cancer (CD133+), malignant melanoma (CD133+), colorectal cancer (EpCAM high / CD44+), colon adenocarcinoma (CD44 high ), glioma (ABCG-2 / BCRP1), digestive tract tumor (ABCG-2 / BCRP1), nasopharyngeal carcinoma (ABCG2), brain glioma (Dlk-1 / Pref-1), gastric cancer (CD45+), Clinical specimens of patients with lung adenocarcinoma (Sca-1 / CD45- / Pecam- / CD34+) and lung cancer (CD133+CD34+CD44+...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com