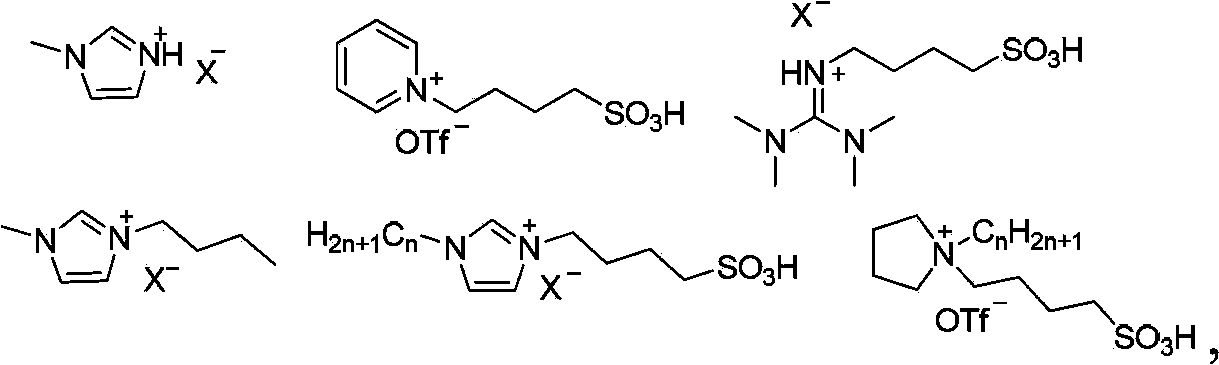
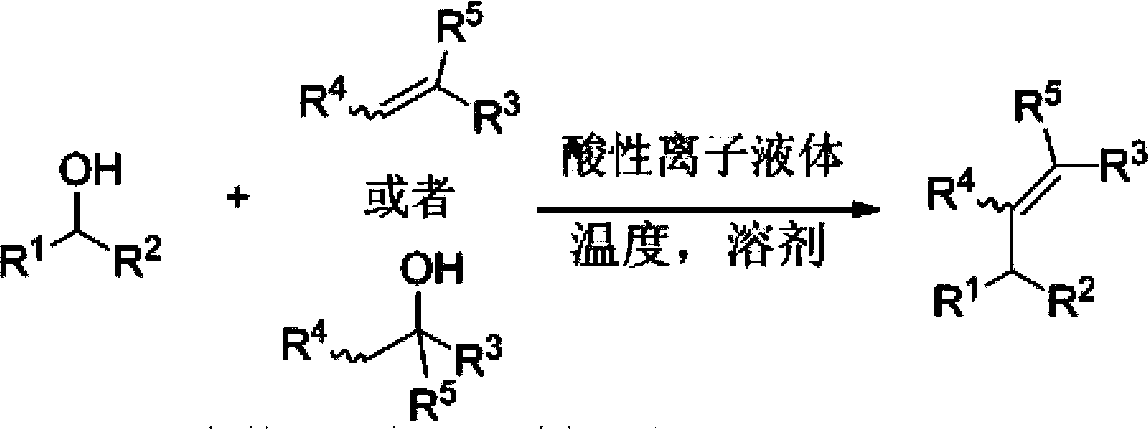
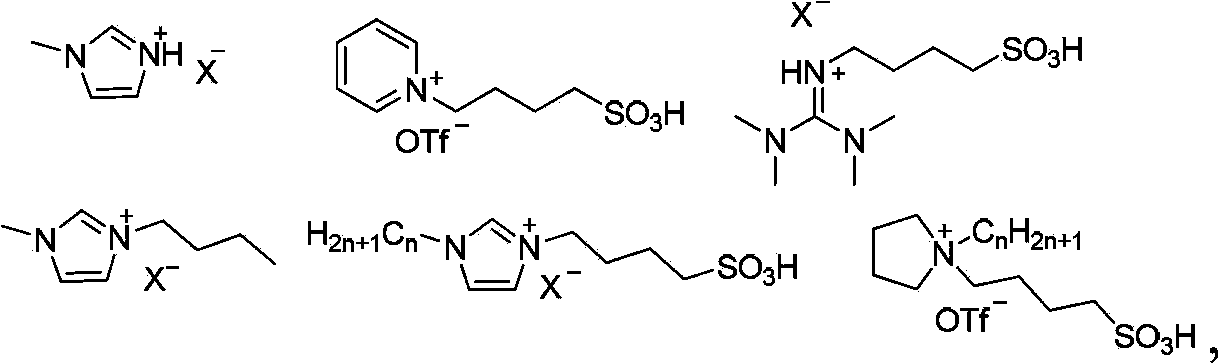
Synthetic method for olefin compounds
A synthetic method and compound technology, applied in organic chemical methods, chemical instruments and methods, and hydrocarbon production from oxygen-containing organic compounds, etc., can solve the problems of harsh reaction conditions, many side reactions, and long reaction time, and achieve simple operation, Mild reaction and low corrosion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] At 80°C, put α-phenylethanol (1.0mmol, 122mg) and 4-chlorobenzhydryl alcohol (0.5mmol, 109.3mg) and [BsOdP]OTf (10mol%, 30.5mg), dichloromethane 2.0mL in a dry place In the reaction flask, magnetically stirred, and reacted for 12h. After the reaction, column chromatography separation (using silica gel column; eluent: petroleum ether) to obtain pure product: (E)-(3-(p-chlorophenyl)-1,3-diphenyl-1-propene , 121.6 mg, the yield was 80%.
Embodiment 2
[0052] At 80°C, α-phenylethanol (1.0mmol, 122mg) and 4-chlorobenzhydryl alcohol (0.5mmol, 109.3mg) and [BsMIm][HSO 4 ] (10mol%, 30.5mg), dichloromethane 2.0mL was placed in a dry reaction flask, magnetically stirred, and reacted for 12h. After the reaction, column chromatography separation (using silica gel column; eluent: petroleum ether) to obtain pure product: (E)-(3-(p-chlorophenyl)-1,3-diphenyl-1-propene , and the yield was 31%.
Embodiment 3
[0054] At 80°C, put α-phenylethanol (1.0mmol, 122mg) and 4-chlorobenzhydryl alcohol (0.5mmol, 109.3mg) and [BsMIm][OTf] (10mol%, 30.5mg), dichloromethane 2.0mL In a dry reaction vial, stirred by magnetic force, reacted for 12h. After the reaction, column chromatography separation (using silica gel column; eluent: petroleum ether) to obtain pure product: (E)-(3-(p-chlorophenyl)-1,3-diphenyl-1-propene , the yield is 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com