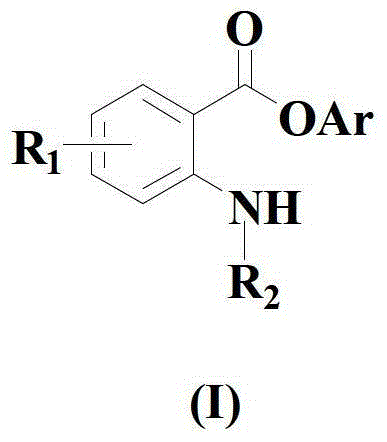
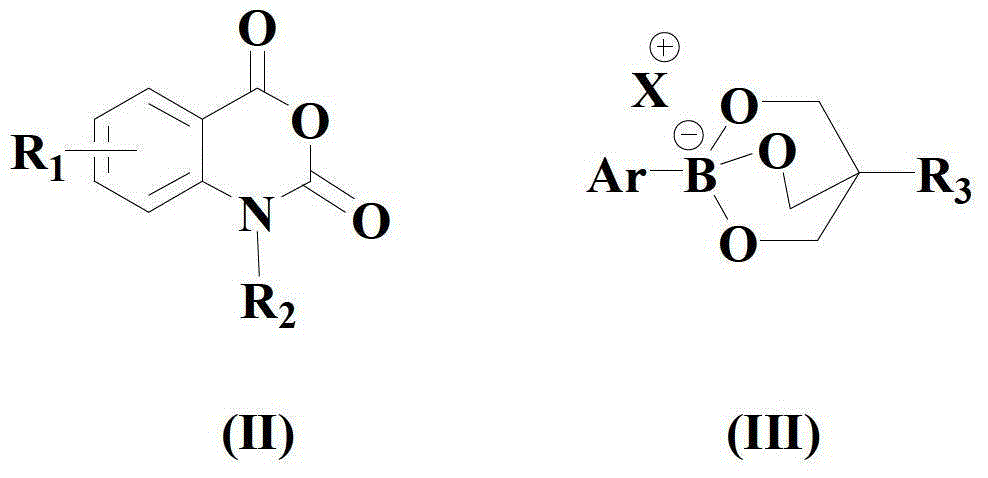A kind of synthetic method of amino-substituted aryl ester compound
A synthesis method and compound technology, which are applied in the synthesis of amino-substituted aryl ester compounds and the field of synthesis of aryl ester compounds, can solve the problems of low yield, further improvement in selectivity, and limitation of practical application, so as to avoid the problem of precious metals Effects of use, good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of phenyl anthranilate
[0061]
[0062] In a dry and clean reactor, add 50ml solvent THF, then add the above formula (II) compound, formula (III) compound, formula (a) copper compound and 1,10-phenanthroline in sequence, so that the molar ratio is 1 :1:0.05:0.1, wherein the compound of formula (II) is 10mmol, and the reaction system is stirred and reacted at 40°C for 15 hours.
[0063] After the reaction was finished, filter, and the filtrate was rotary evaporated with a rotary evaporator to remove the solvent, and the residue was purified by 200-300 mesh silica gel column chromatography to obtain the target product as a solid, with a yield of 99.4% and a purity of 99.1% ( HPLC).
[0064] Melting point: 70-71°C;
[0065] NMR: 1 H NMR (500MHz, CDCl 3 )δ5.76(s,2H),6.67-6.72(m,2H),7.14-7.20(m,2H),7.27-7.29(m,1H),7.30-7.35(m,1H),7.39-7.45( tm,2H), 8.07-8.10(m,1H).
Embodiment 2
[0066] Embodiment 2: the synthesis of m-methylphenyl anthranilic acid
[0067]
[0068] In a dry and clean reactor, add 50ml of solvent acetone, then add the compound of formula (II), compound of formula (III), copper compound of formula (b) and 1,10-phenanthroline monohydrate successively to make the mole The ratio is 1:1.5:0.1:0.2, wherein the compound of formula (II) is 10 mmol, and the reaction system is stirred and reacted at 50° C. for 20 hours.
[0069] After the reaction was completed, filter, and the filtrate was evaporated with a rotary evaporator to remove the solvent, and the residue was purified by 300-400 mesh silica gel column chromatography to obtain the target product with a yield of 97.6% and a purity of 98.2% (HPLC).
[0070] NMR: 1 H NMR (500MHz, CDCl 3 )δ2.41(s,3H),5.76(s,2H),6.70-6.75(m,2H),6.98-7.03(m,2H),7.07-7.11(m,1H),7.31-7.37(m, 2H), 8.09-8.11 (m, 1H).
Embodiment 3
[0071] Embodiment 3: the synthesis of p-methoxyphenyl anthranilic acid
[0072]
[0073] In a dry and clean reactor, add 50ml of solvent HMPA, then add the above formula (II) compound, formula (III) compound, formula (a) copper compound and 1,10-phenanthroline in turn, so that the molar ratio is 1 :2:0.15:0.3, wherein the compound of formula (II) is 10mmol, and the reaction system is stirred and reacted at 60°C for 25 hours.
[0074] After the reaction was finished, filter, and the filtrate was rotary evaporated with a rotary evaporator to remove the solvent, and the residue was purified by 400-500 mesh silica gel column chromatography to obtain the target product as a solid, with a yield of 76.7% and a purity of 97.9% ( HPLC).
[0075] Melting point: 102-103°C;
[0076] NMR: 1 H NMR (500MHz, CDCl 3 )δ3.85(s,3H),5.78(s,2H),6.72(d,J=7.8Hz,2H),6.94(d,J=7.2Hz,2H),7.08-7.12(m,2H), 7.30-7.36 (m, 1H), 8.09-8.11 (m, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com