High-selectivity catalyst for preparing phthalide by hydrogenation of phthalic anhydride
A highly selective, catalyst technology, used in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, organic chemistry, etc., can solve the limitation of active ingredient loading, poor stability, and difficult preparation It solves the problems of high dispersibility and high loading of catalysts, and achieves the effects of high selectivity, easy availability of raw materials, and good industrial application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Ni / Al 2 o 3 Catalyst preparation
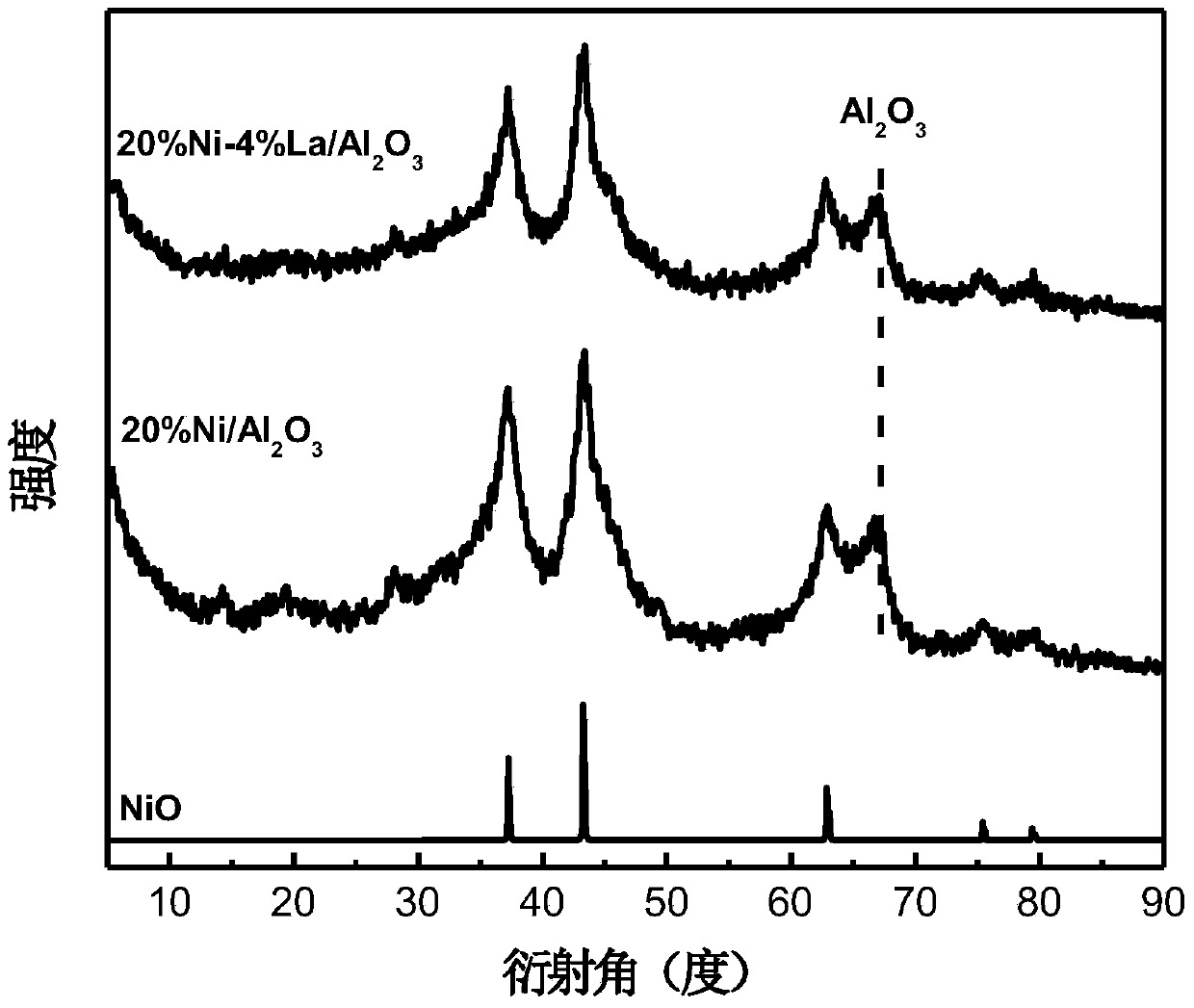
[0020] Co-precipitation method was used to prepare 0.75mol / L Ni(NO 3 ) 2 ·6H 2 O and 0.25mol / L Al(NO 3 ) 3 9H 2 O solution A50mL, prepared to contain 0.80mol / L Na 2 CO 3 and 1.20mol / L NaOH alkali solution B50mL, at 65°C, add solution A evenly dropwise into solution B, maintain the pH of the reaction system at 9-10, stir vigorously, and co-precipitate. After the titration is completed, continue to stir and age, and finally filter, wash, and dry to obtain a nickel-containing hydrotalcite precursor. The obtained nickel-containing hydrotalcite precursor is calcined at 500°C and reduced at 400°C for 2 hours to obtain Ni / Al 2 o 3 catalyst. figure 1 Ni / Al is obtained by roasting the nickel-containing hydrotalcite material as the precursor 2 o 3 X-ray diffraction pattern of the catalyst.
Embodiment 2
[0021] Embodiment 2: the preparation of Co / ZnO catalyst
[0022] Co-precipitation method was used to prepare 0.75mol / L Co(NO 3 ) 2 ·6H 2 O and 0.25mol / L Zn(NO 3 ) 3 ·6H 2 O solution A50mL, prepared to contain 0.80mol / L Na 2 CO 3 and 1.20mol / L NaOH alkali solution B50mL, at 65°C, add solution A evenly dropwise into solution B, maintain the pH of the reaction system at 9-10, stir vigorously, and co-precipitate. After the titration is completed, continue to stir and age, and finally filter, wash, and dry to obtain a nickel-containing hydrotalcite precursor. The obtained nickel-containing hydrotalcite precursor is calcined at 500°C and reduced at 400°C for 2 hours to obtain a Co / ZnO catalyst.
Embodiment 3
[0023] Example 3: NiAu / Al 2 o 3 Catalyst preparation
[0024] Co-precipitation method was used to prepare 0.70mol / L Ni(NO 3 ) 2 ·6H 2 O, 0.05mol / L chloroauric acid and 0.25mol / L Al(NO 3 ) 3 9H 2 O solution A50mL, prepared to contain 0.80mol / L Na 2 CO 3and 1.20mol / L NaOH alkali solution B50mL, at 65°C, add solution A evenly dropwise into solution B, maintain the pH of the reaction system at 9-10, stir vigorously, and co-precipitate. After the titration is completed, continue to stir and age, and finally filter, wash, and dry to obtain a nickel-gold-containing hydrotalcite-like precursor. The obtained nickel-gold-containing hydrotalcite-like precursor was calcined at 500°C and reduced at 400°C for 2 hours to obtain NiAu / Al 2 o 3 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com