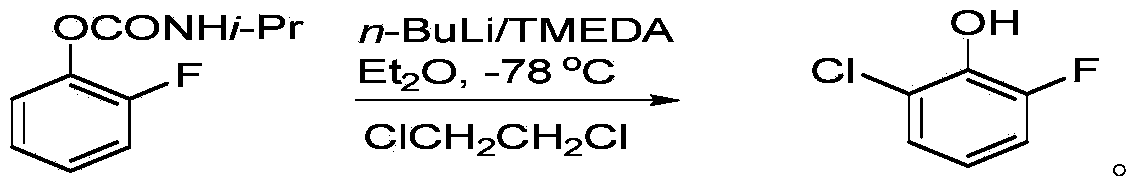
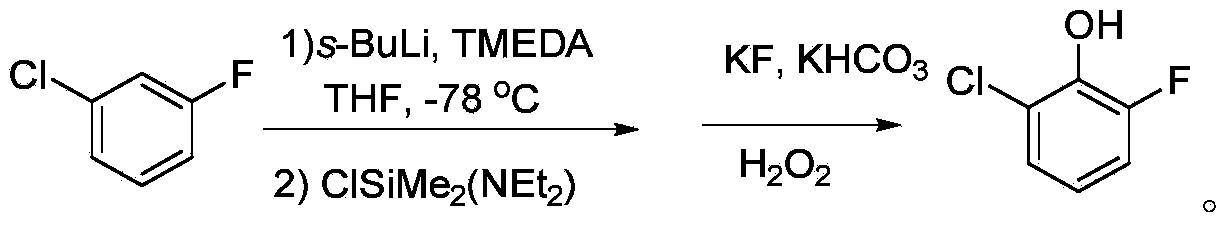
Preparation method for 2-chloro-6-fluorophenol
A technology of fluorophenol and o-fluorophenol, applied in the field of preparation of 2-chloro-6-fluorophenol, can solve the problems of low reaction temperature, high price, inability to operate and produce in large quantities, etc., and achieves the effects of simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] ⑴Add 10mL CH to a 100mL three-necked bottle 2 Cl 2 and 2.0g (0.018mol) o-fluorophenol, slowly drop 20mL (0.032mol) NaClO solution (active chlorine 5.68g / 100mL, 1.6mol / L) into the there-necked flask through the dropping funnel, the color gradually changes during the dropping process Deep; the reaction temperature is kept at about 40°C, and GC tracking is performed every 30 minutes. After 1 hour of reaction, GC tracking confirms that the reaction is terminated; (2) Add 2-6mol / L dilute hydrochloric acid to the three-necked flask to neutralize the reaction, so that the reaction solution becomes weak acidic, adjust the pH to be 6, and stand to separate the organic phase; (3) the organic phase separated in step (2) is washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent is removed by rotary evaporation under reduced pressure to obtain a crude product, and the crude product is then The product 2-chloro-6-fluorophenol was...
Embodiment 2
[0024] In this example, 16 mL of NaClO solution was used in step (1) to replace 20 mL of NaClO solution in step (1) of Example 1, and the remaining operations were the same as in Example 1 to obtain 2.1 g of the product 2-chloro-6-fluorophenol with a yield of 80% , 98.3% purity.
Embodiment 3
[0026] In the present embodiment, use 15mLCCl in step (1) 4 10mLCH in alternative embodiment 1 step (1) 2 Cl 2 , the remaining operations were the same as in Example 1 to obtain 2.0 g of the product 2-chloro-6-fluorophenol, with a yield of 76.5% and a purity of 98%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap