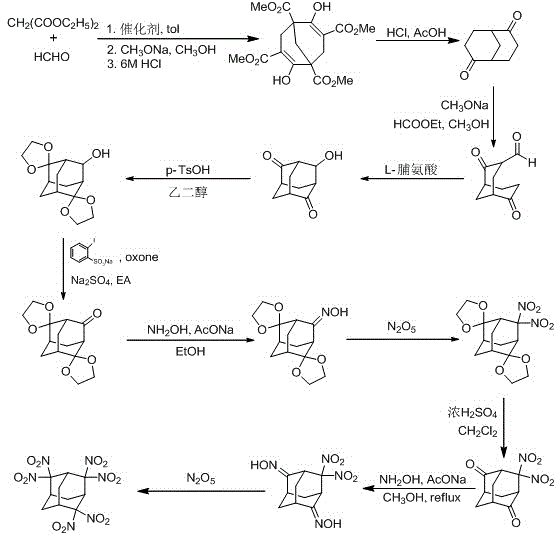
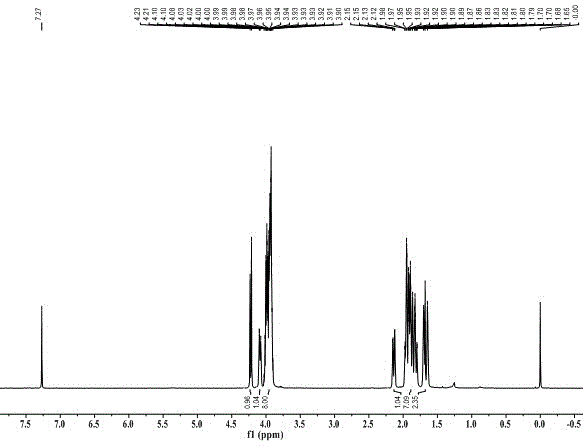
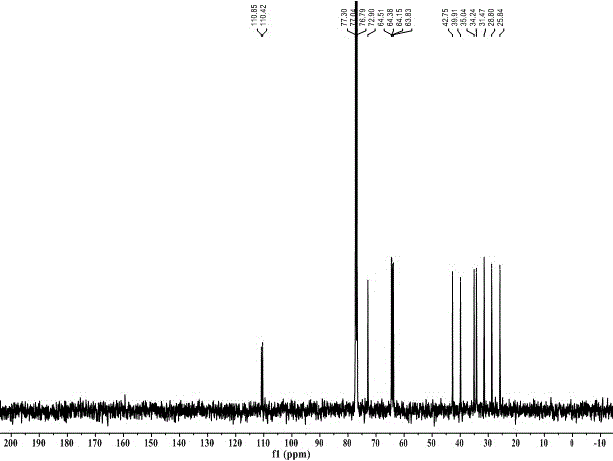
Synthesis method for 2,2,4,4,6,6-hexanitro-adamantane
A synthetic method, the technology of the six nitrate foundation, applied in chemical instruments and methods, the preparation of organic compounds, organic chemistry, etc., can solve the problems of many waste acids, long steps, low yield, etc., and achieve high reaction yield and low cost. Low, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Preparation of Meerwein's ester
[0042]
Embodiment 1
[0044] Add 228mL (1.50mol) of diethyl malonate, 37.5g (1.25mol) of paraformaldehyde, and 200mL of toluene into a 500mL round-bottomed flask in turn, and add 4.2mL (0.038mol) of N-methylpiperazine while stirring, first React at 100°C for 8h, then at 120°C for 10h. The solvent was distilled off under reduced pressure to obtain a light yellow oily liquid, which was cooled to room temperature and set aside. Add 57.4g (1.06mol) of sodium methoxide into 400mL of anhydrous methanol, stir until completely dissolved, and after cooling to room temperature, add the light yellow oily liquid obtained above to it. After reflux at 65°C for 12 hours, a light yellow solid precipitated out. After cooling to room temperature, it was filtered with suction, and the filter cake was washed with a mixed solvent of ether and methanol to obtain a white solid. Dissolve the white solid in 500 mL of water, adjust the pH to 4-5 with 6 mol / L hydrochloric acid, precipitate a large amount of white solid, fil...
Embodiment 2
[0046] Only change the amount of paraformaldehyde in Example 1 to 31.5g (1.05mol), change the reaction to first react at 80°C for 9h, change the amount of sodium methoxide to 81.2g (1.50mol), and other operations are consistent with Example 1 . Obtain 80.8g Meerwein's ester, yield 56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com