Method for efficiently expressing L-asparaginase II by recombinant escherichia coli
A technology of recombinant Escherichia coli and asparaginase, which is applied in the biological field and can solve the problem of low enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
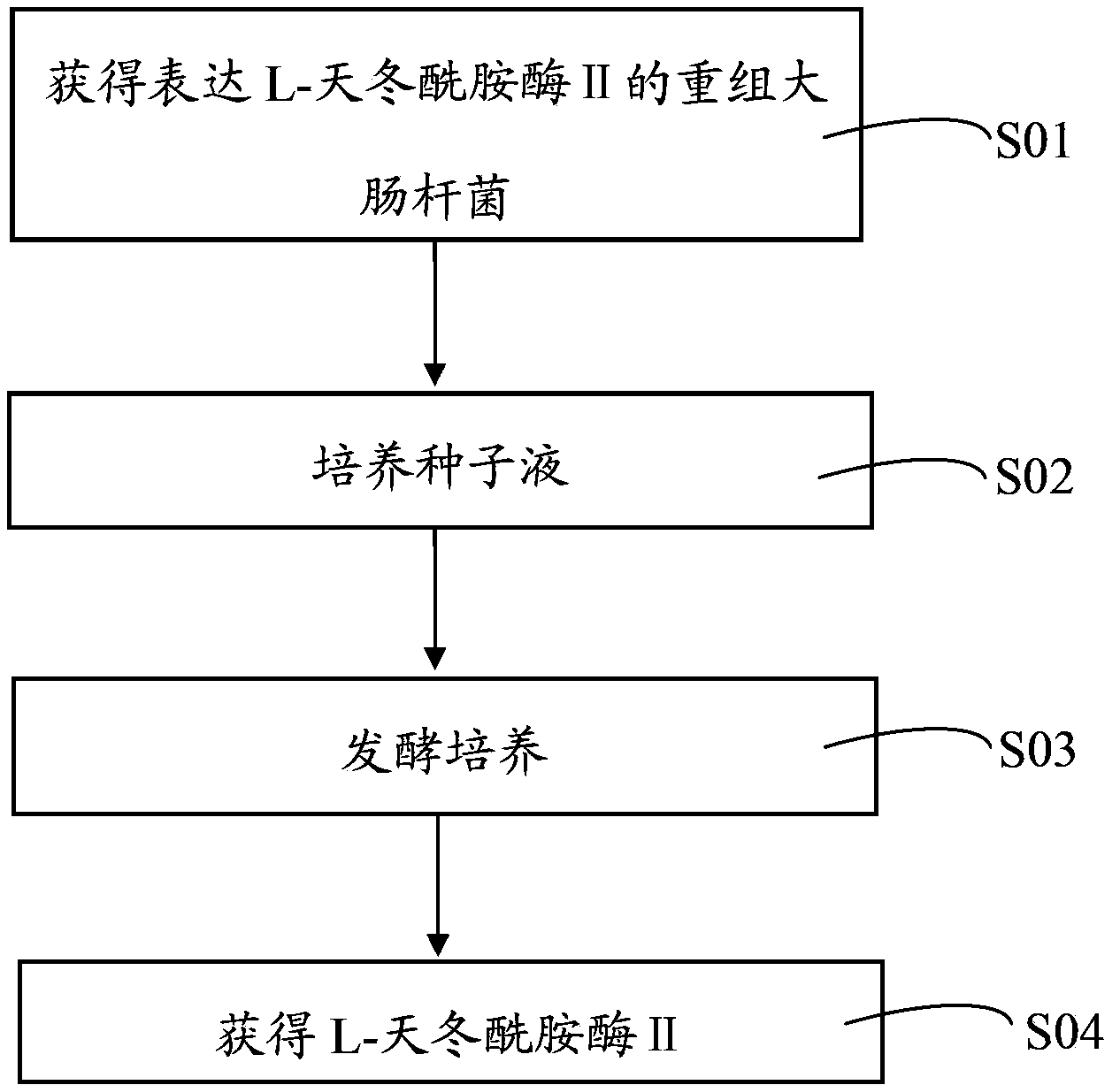
[0037] Recombinant Escherichia coli for efficient expression of L-asparaginase Ⅱ, see figure 1 As shown, including the following steps:
[0038] S11. Obtain the recombinant E. coli expressing L-asparaginase II:
[0039] (1) Amplify gene fragments: design and synthesize primers, where the upstream primer is 5'TGC GGATCC CAT TAC CCA ATA TCA-3', downstream primer is 5'GAG CTCGAG GTA CTG ATT GAA CT3', extract DNA from E. coli JM109 as a template, mix the above primers and amplification template into a set of reaction tubes, perform PCR amplification, and obtain amplified gene fragments;
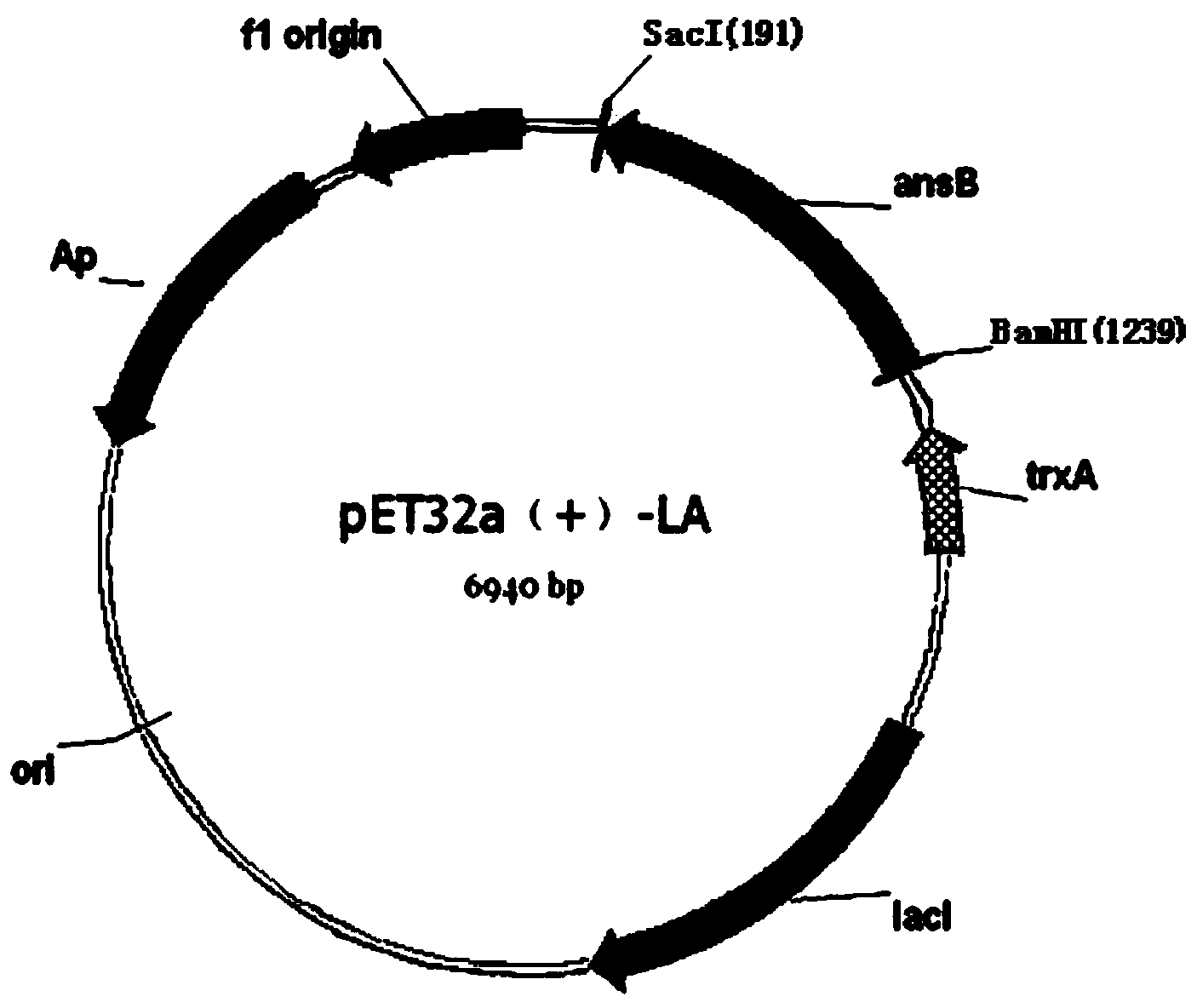
[0040] (2) Construction of recombinant plasmid: select plasmid pET-32a (+) as the vector for transformation, and simultaneously digest the amplified gene fragment and plasmid pET-32a (+) with endonucleases BamHI and Sac I, and then use T4 ligase to The amplified gene fragments digested with the same double enzymes were ligated with plasmid pET-32a(+) to obtain recombinant plasmid pET32a(+)-LA, and th...
Embodiment 2
[0051] Recombinant Escherichia coli for efficient expression of L-asparaginase Ⅱ, see figure 1 As shown, including the following steps:
[0052] S21. Obtain the recombinant Escherichia coli expressing L-asparaginase II, and the method is the same as that of S11 in Example 1, which will not be repeated here.
[0053] S22. The method of cultivating the seed liquid is the same as that of S12 in Example 1, and will not be repeated here.
[0054] S23. Fermentation culture: The engineered bacteria obtained after culturing the seed liquid obtained in step S12 overnight is used as the fermentation liquid, and 7% of the total inoculation amount is connected to medium 2 and put into the fermentation tank for stepwise fermentation culture:
[0055] (1) The initial parameter setting of the fermenter reaction is the same as S13(1) of embodiment 1, and will not be repeated here;
[0056] (2) When the culture reaches 5h, set the culture temperature to 32℃, the conditions for lactose induction are the ...
Embodiment 3
[0061] Recombinant Escherichia coli for efficient expression of L-asparaginase Ⅱ, see figure 1 As shown, including the following steps:
[0062] S31. Obtain the recombinant Escherichia coli expressing L-asparaginase II. The method is the same as that of S11 in Example 1, which will not be repeated here.
[0063] S32. The method for cultivating the seed liquid is the same as that of S12 in Example 1, and will not be repeated here.
[0064] S33: Fermentation culture: The engineered bacteria obtained after culturing the seed liquid obtained in step S12 overnight is used as the fermentation liquid, and 7% of the total inoculation amount is connected to medium 2 and put into the fermentor for stepwise fermentation culture:
[0065] (1) The initial parameter setting of the fermenter reaction is the same as that of S13(1) in Example 1, and will not be repeated here;
[0066] (2) When cultured to 5h, keep the culture temperature at 37℃, the conditions of lactose induction are the same as S13(2)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com