Synthesis method for 5-substituted benzofuran-2-carboxylic acid and derivatives thereof
A synthetic method, the technology of phenylpropanoid furan, which is applied in the direction of organic chemistry, can solve the problems of high cost, harsh production conditions, unsuitable for industrial production, etc., and achieve the effects of low production cost, good product quality and great practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
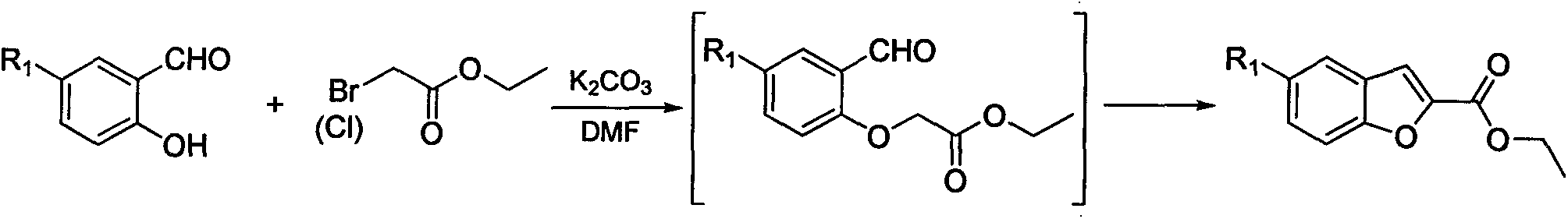
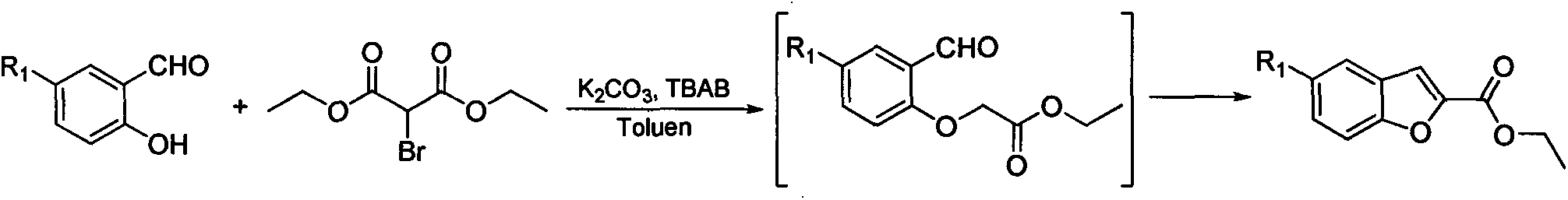
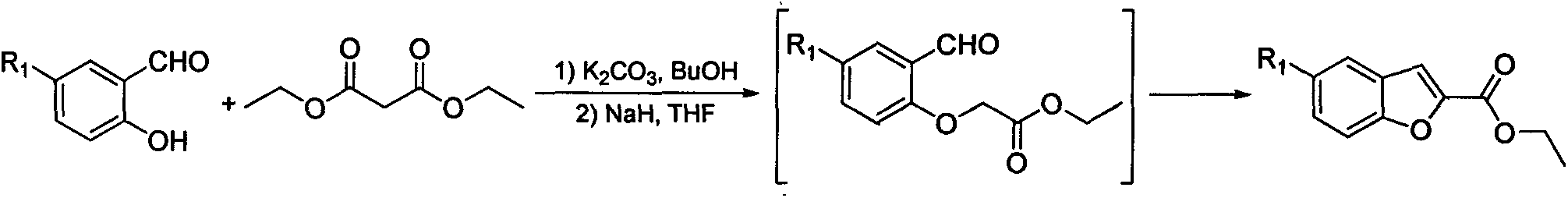
Image
Examples
Embodiment 15
[0030] The synthesis of embodiment 15-acetyl phenylpropanoid-2-carboxylate ethyl ester
[0031] Add 136.1g of p-acetylphenol and 1L of concentrated hydrochloric acid into a 2L three-neck flask, then add 300mL of 37% formaldehyde solution, and react at 50°C for 4h while stirring. After cooling to room temperature, the resulting red precipitate was filtered, washed with water, and dried to obtain 160.5 g of red solid which was 2-chloromethyl-4-acetylphenol, HPLC > 97%, crude product yield 86.9%. The crude product can be directly used in the next step without further purification, and a white analytically pure sample can be obtained by recrystallization from toluene or THF.
[0032] 1 H NMR (DMSO-d 6 , 400MHz): δ2.51(s, 3H, CH 3 ), 4.74 (s, 2H, CH 2 ), 6.98 (d, J=8.4Hz, 1H, BzH), 7.83 (d, J=8.4Hz, 1H, BzH), 8.01 (s, 1H, BzH), 10.85 (brs, 1H, OH).
[0033] 160g of 2-chloromethyl-4-acetylphenol and 227g of triphenylphosphine obtained in the previous step were stirred and reflu...
Embodiment 2
[0037]Embodiment 2 benzofuran-2, the synthesis of 5-dicarboxylate diethyl ester
[0038] Put 1L of hydrobromic acid into a 2L three-necked flask, then add 138.1g of p-acetylphenol and 68.5g of paraformaldehyde, and react at 60-70°C for 2 hours while stirring. Then cooled to room temperature, the resulting brown-red precipitate was filtered, washed with water, and dried to obtain 212.3 g of a red solid which was 2-bromomethyl-4-carboxyphenol, HPLC > 96%, and the yield of the crude product was 91.9%. The crude product can be directly used in the next step without further purification, and recrystallization from ethyl acetate or tetrahydrofuran can give a pale yellow analytically pure sample.
[0039] 1 H NMR (DMSO-d 6 , 400MHz): δ4.58(s, 2H, CH 2 ), 7.13 (d, J=8.4Hz, 1H, BzH), 7.98 (d, J=8.4Hz, 1H, BzH), 8.17 (s, 1H, BzH), 10.87 (brs, 1H, OH).
[0040] 210g of 2-bromomethyl-4-carboxyphenol and 238.5g of triphenylphosphine obtained in the previous step were refluxed in 1.5L o...
Embodiment 35
[0044] The synthesis of embodiment 35-chlorobenzofuran-2-carboxamide
[0045] Put 1L of concentrated hydrochloric acid into a 2L three-necked flask, then add 128.6g of p-chlorophenol and 77.1g of paraformaldehyde, and react at 60-70°C for 3h while stirring. Then cooled to room temperature, the resulting yellow precipitate was filtered, washed with water, and dried to obtain 147.6 g of a yellow solid which was 2-chloromethyl-4-chlorophenol, HPLC>96%, and the yield of the crude product was 83.4%. The crude product can be directly used in the next step without further purification, and a white analytically pure sample can be obtained by recrystallization from ethyl acetate or THF.
[0046] 1 H NMR (DMSO-d 6 , 400MHz): δ4.63(s, 2H, CH 2 ), 6.91 (d, J=8.0Hz, 1H, BzH), 7.38 (d, J=8.0Hz, 1H, BzH), 7.48 (s, 1H, BzH), 10.12 (brs, 1H, OH).
[0047] 145g of 2-chloromethyl-4-chlorophenol and 215g of triphenylphosphine obtained in the previous step were refluxed in 1L of toluene for 4h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com