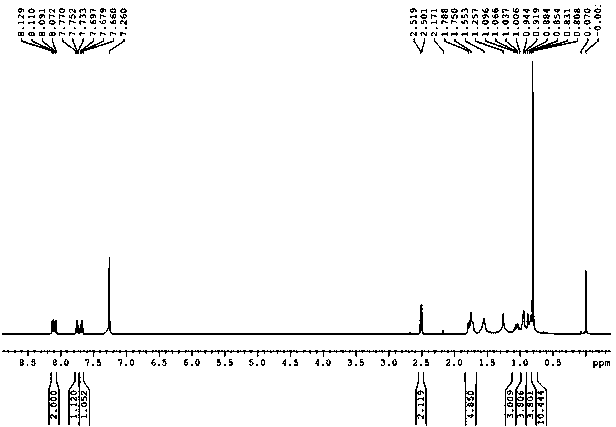
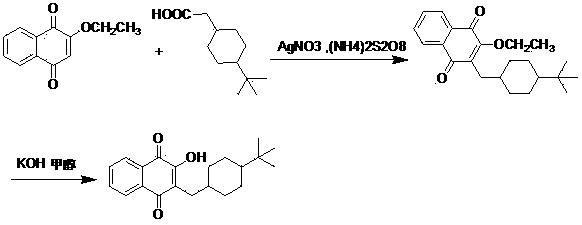
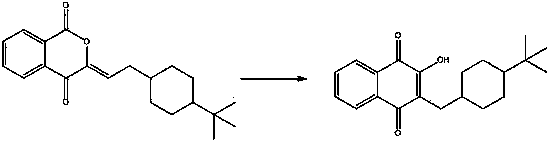
Benzopyranone compound, as well as preparation method and application thereof
A technology of benzopyrone and compound, applied in the field of pharmaceutical synthesis, can solve problems such as low yield, and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment one Preparation of 3-[(4-tert-butylcyclohexyl) dimethylene]-1,4-benzopyrandione
[0043] Under argon protection, add 1.62g (0.01mol) of 1,4-benzopyrandione to a 50ml three-necked flask, slowly add 20ml of acetic acid solution of 1.82g (0.01mol) of p-tert-butylcyclohexylaldehyde, and add 0.87ml (0.01mol) of isobutylamine was heated to 60°C for 4h. 20ml of water was added dropwise, solids were precipitated, filtered by suction, and recrystallized with 50ml of methyl tert-butyl ether to finally obtain 2.14g of a yellow solid with a yield of 65.6%.
Embodiment 2
[0044] Embodiment two Preparation of 3-[(4-tert-butylcyclohexyl) dimethylene]-1,4-benzopyrandione
[0045] Under the protection of nitrogen, add 3.24g (0.02mol) of 1,4-benzopyrandione into a 100ml three-necked flask, slowly add 20ml of acetic acid solution of 7.48g (0.04mol) of p-tert-butylcyclohexylaldehyde, and add iso Butylamine 1.74ml (0.02mol), heated to 30°C for 10h. 40ml of water was added dropwise, solids were gradually precipitated, and the crude product was obtained by suction filtration.
[0046] It was recrystallized with 100ml of diethyl ether and dried under vacuum at 50°C to obtain 4.22g of a yellow solid with a yield of 64.8%.
Embodiment 3
[0047] Embodiment Three Preparation of 3-[(4-tert-butylcyclohexyl) dimethylene]-1,4-benzopyrandione
[0048] Under argon protection, add 1.62g (0.01mol) of 1,4-benzopyrandione to a 50ml three-necked flask, slowly add 20ml of acetic acid solution of 1.82g (0.01mol) of p-tert-butylcyclohexylaldehyde, and add Morpholine 2.61ml (0.03mol), warmed up to 40°C for 4h. 20ml of water was added dropwise, solids were gradually precipitated, and the crude product was obtained by suction filtration.
[0049] Recrystallized with 50ml of isopropyl ether, dried in vacuo at 70°C to obtain 2.07g of yellow solid, yield 63.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com